Fig. 2.
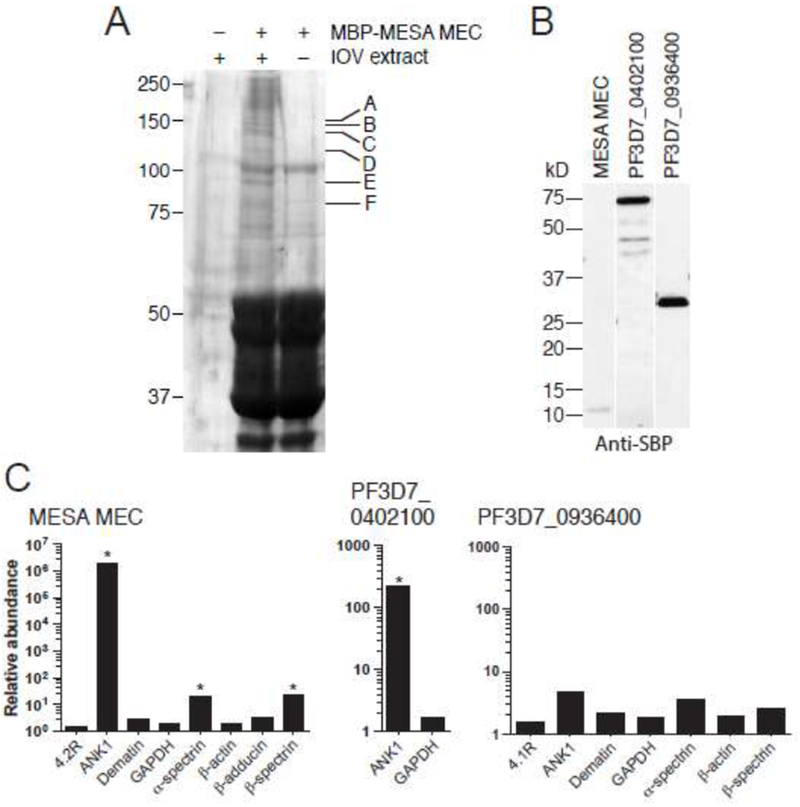
A. Co-affinity purification of erythrocyte cytoskeletal proteins with the MESA MEC motif. MBP-MESA was expressed in E. coli, purified, incubated with LIS-extracted erythrocyte ghosts, and purified again on amylose beads. Eluted proteins were subjected to SDS-PAGE and visualized by Coomassie blue staining. Bands found specifically in the MBP-MESA plus erythrocyte extract sample (indicated by letters) were excised from the gel, digested with trypsin, and subjected to mass spectrometry (see Table 1 for protein identification).
B. Expression of SBP-tagged P. falciparum proteins. PF3D7_0402100, and PF3D7_0402100. C-terminal SBP-tagged MESA MEC motif, PF3D7_0402100, (minus the region N-terminal to the predicted export motif) and PF3D7_0936400 (does not bind to IOVs) were in vitro translated in WGE, subjected to SDS-PAGE followed by western blotting with anti-SBP antibody.
C. Co-purification of erythrocyte cytoskeletal proteins with SBP-tagged P. falciparum proteins. In vitro translated SBP-tagged proteins were incubated with a soluble erythrocyte cytoskeletal extract and purified using streptavidin-coated beads. Co-purifying proteins were identified by LC-MS/MS. The plot shows the relative abundance of erythrocyte proteins that co-purified compared to the negative control (SBP-tagged GFP), which was set at 1 (* SAINT score >0.9).
