Fig. 3.
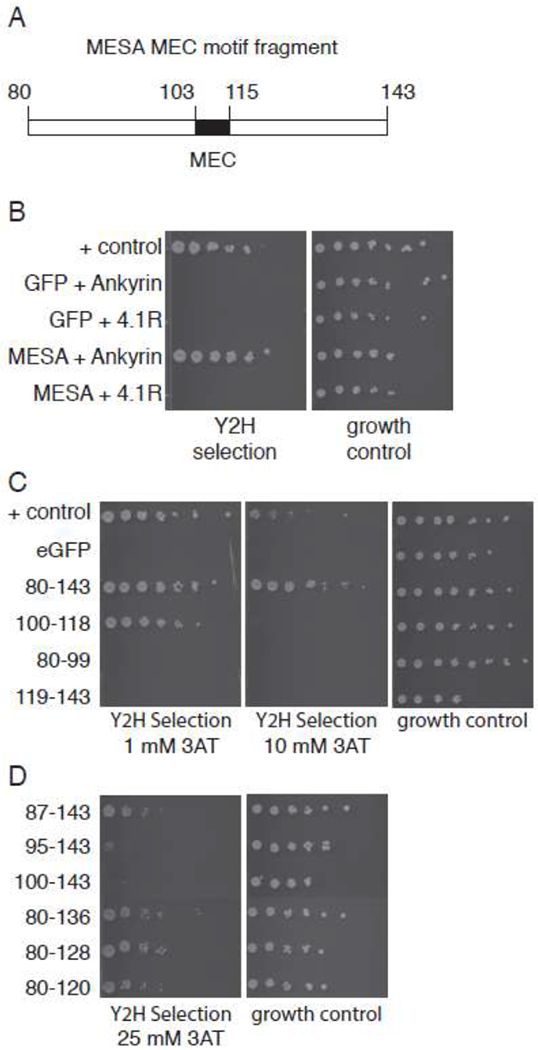
The MESA MEC motif interacts with erythrocyte ankyrin in the yeast two-hybrid assay.
A. MESA MEC fragment for yeast two-hybrid experiments. Numbers indicate amino acid positions in full length MESA.
B. Yeast two-hybrid assays. Diploid yeast expressing GFP or MESA MEC motif as fusions to the Gal4 activation domain (AD) and ankyrin or 4.1R as fusions to the Gal4 DNA binding domain (BD) were plated on yeast two-hybrid selection medium and medium lacking tryptophan and leucine (to show equal numbers of cells). Positive control cells express PFE1350c and PFC0255c as fusions to Gal4 AD and DBD.
C. MESA MEC motif N- and C-terminal flanking regions do not bind to ankyrin. Subfragments of the MESA MEC motif construct in (A) containing the MEC motif or the N- or C-terminal flanking regions were tested for binding to ankyrin in the yeast two-hybrid assay as in part B, except that the 3-AT concentrations were 1 and 10 mM in the left and middle panels, respectively. Higher 3-AT concentrations are more stringent.
D. Binding of MESA MEC motif truncation mutants to ankyrin. Six N- and C-terminal deletions of the MESA MEC motif construct were tested for binding to ankyrin in the yeast two-hybrid assay. Five-fold serial dilutions of diploid yeast were plated on yeast two-hybrid selection medium containing 25 mM 3-AT or medium lacking tryptophan and leucine.
