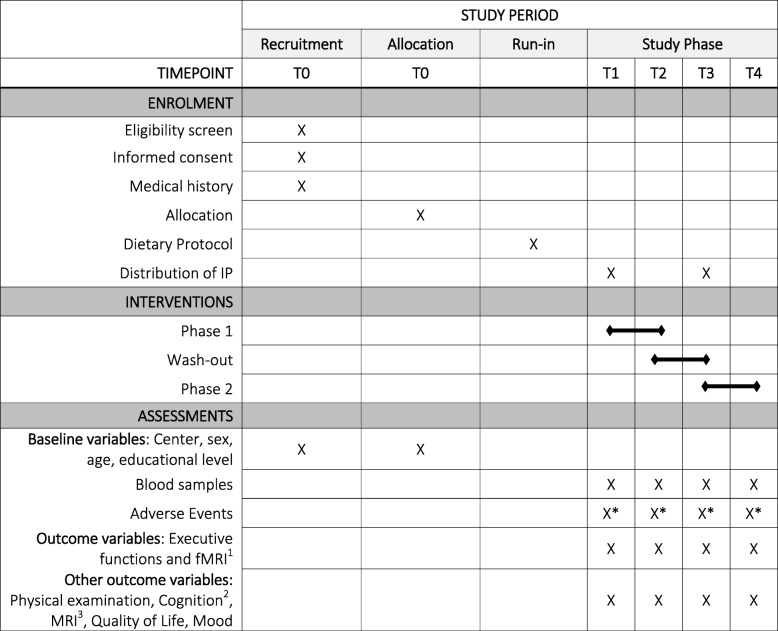
Table 1.
Schedule of enrolment, interventions, and assessment (according to the SPIRIT guidelines)
T0 = Recruitment, Informed Consent, Randomization; T1 = before the first treatment arm (neuropsychology, neuroimaging and blood sample assessment); T2 = after the first treatment arm (neuropsychology, neuroimaging and blood sample assessment); T3 = after the wash-out period and before the second treatment arm (neuropsychology, neuroimaging and blood sample assessment); T4 = after the second treatment arm (neuropsychology, neuroimaging and blood sample assessment); IP Investigational product
*additional weekly phone-calls to assess adverse events
1Working memory, inhibition, cognitive flexibility, neural activation during working memory fMRI
2IQ, memory, fine motor speed, attention
3Voxel-based morphometry, diffusion tensor imaging, resting-state functional imaging, arterial spin labeling, magnetic resonance spectroscopy