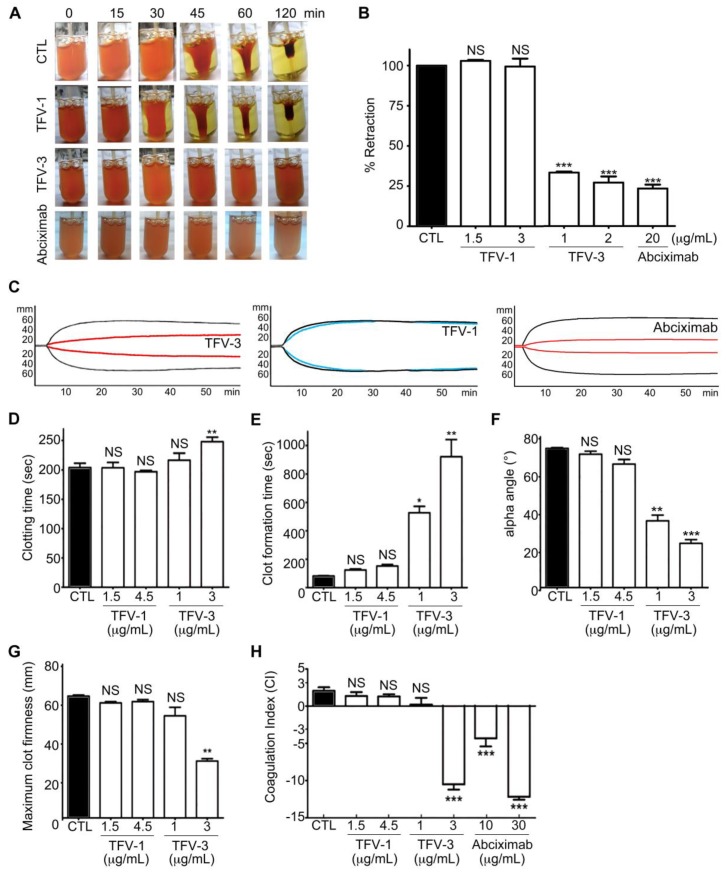
Figure 6.
Effects of TFV-1 and TFV-3 on human hemostasis in vitro. (A,B) Effect of TFV-1 and TFV-3 on thrombin-induced clot retraction in human PRP. PRP was incubated with various concentrations of TFV-1 or TFV-3 at 37 °C for 3 min before addition of thrombin (4 U/mL). To observe the kinetics of clot retraction, photographs were taken at time 0 and every 15 min until 120 min. Percent retraction was measured by the volume of serum (test)/volume of serum (control). These data are presented as mean ± SEM (n = 6). *** p < 0.001 compared with the control group. (C–H) Physiologic platelet functions of TFV1 and TFV3 were evaluated by rotational thromboelastometry (ROTEM) assays. Human whole blood was incubated with TFV-1, TFV-3, or abciximab, and the ROTEM trace (C) of TFV-1 (1.5 μg/mL), TFV-3 (1 μg/mL) and abciximab (10 μg/mL) in human whole blood are shown. CTL (black line): in the absence of agents. Clotting time (D), clot formation time (E), α-angle (F), maximum clot firmness (G) and coagulation index (H) were evaluated with a ROTEM analyzer following recalcification of the blood, to determine clot and coagulation kinetics. Data were presented as means ± SEM (n = 7). * p < 0.05, ** p < 0.01, *** p <0.001 compared with the control group.