Figure 3. Pharmacological Effects on CRS-, Inflammatory-, and Neural Injury-Induced Pain.
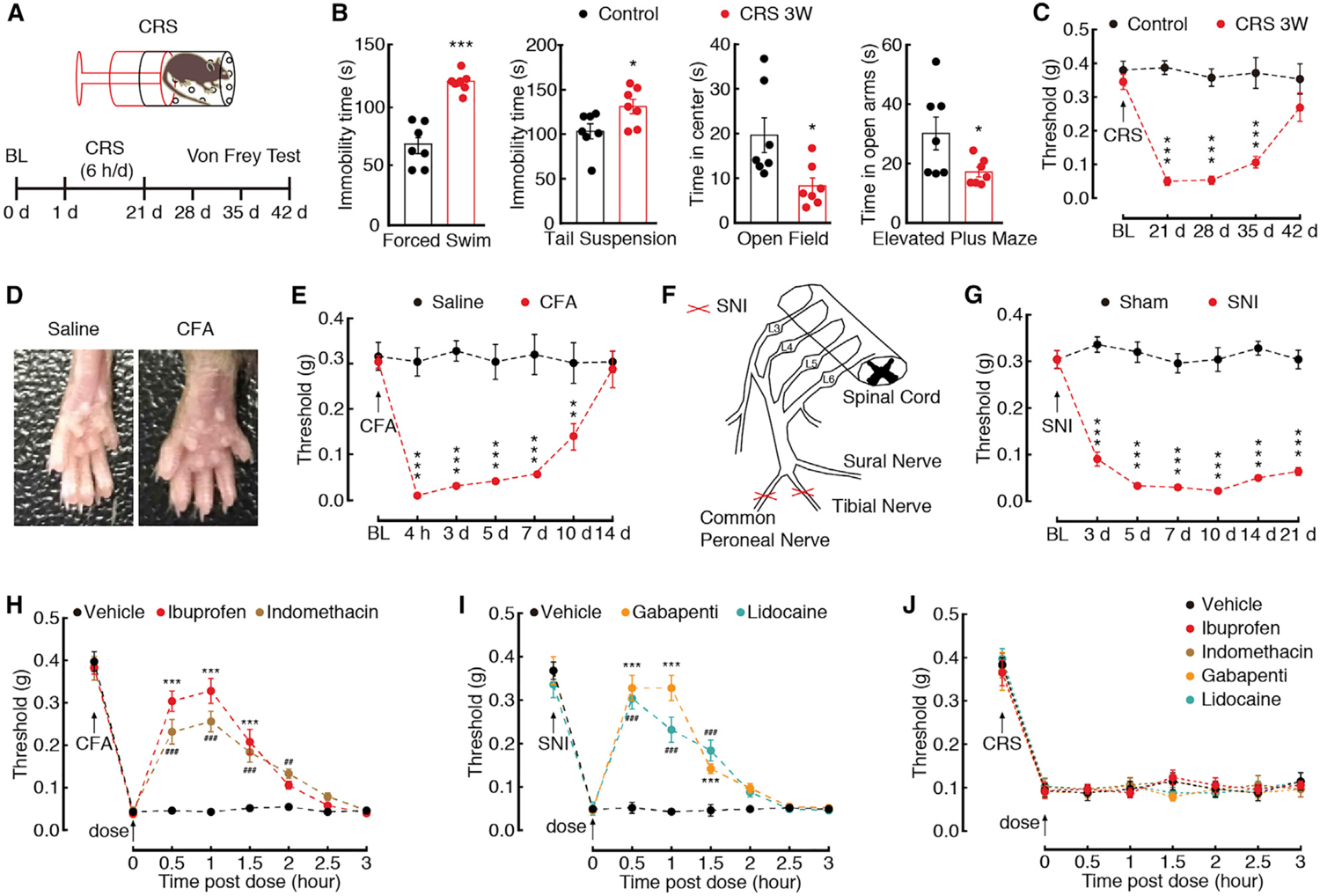
(A) An outline of the experimental procedure for mice with CRS treatment and behavioral tests.
(B) Behavioral tests in forced swim, tail suspension, open field, and elevated plus maze (n = 7 mice per group).
(C) Time course of CRS-induced changes in pain threshold by Von Frey tests (n = 7 mice per group).
(D) The mouse left hind paw at day 3 after saline or CFA injection.
(E) CFA-induced changes in pain threshold (n = 5 mice per group).
(F) Schematic of the animal model of SNI.
(G) SNI-induced changes in pain threshold (n = 5 mice per group).
(H) Effects of ibuprofen (10 mg/kg, i.p.) or indomethacin (10 mg/kg, i.p.) on pain thresholds at day 3 after CFA treatment (n = 5 mice per group). *Preference comparison within the ibuprofen group; #preference comparison within the indomethacin group.
(I) Effects of gabapentin (10 mg/kg, i.p.) or lidocaine (10 mg/kg, i.p.) on pain thresholds at day 7 after SNI (n = 5 mice per group). *Preference comparison within the gabapentin group;, #preference comparison within the lidocaine group.
(J) Effects of ibuprofen, indomethacin, gabapentin, or lidocaine on pain thresholds of CRS 3W mice (n = 5 mice per group).
All data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. ## p < 0.01, ### p < 0.001. Unpaired t test for (B); two-way repeated-measures ANOVA with Bonferroni post hoc analysis for (C), (E), and (G)–(J). See also Figure S4.
