Fig. 4. Structure of BcsG.
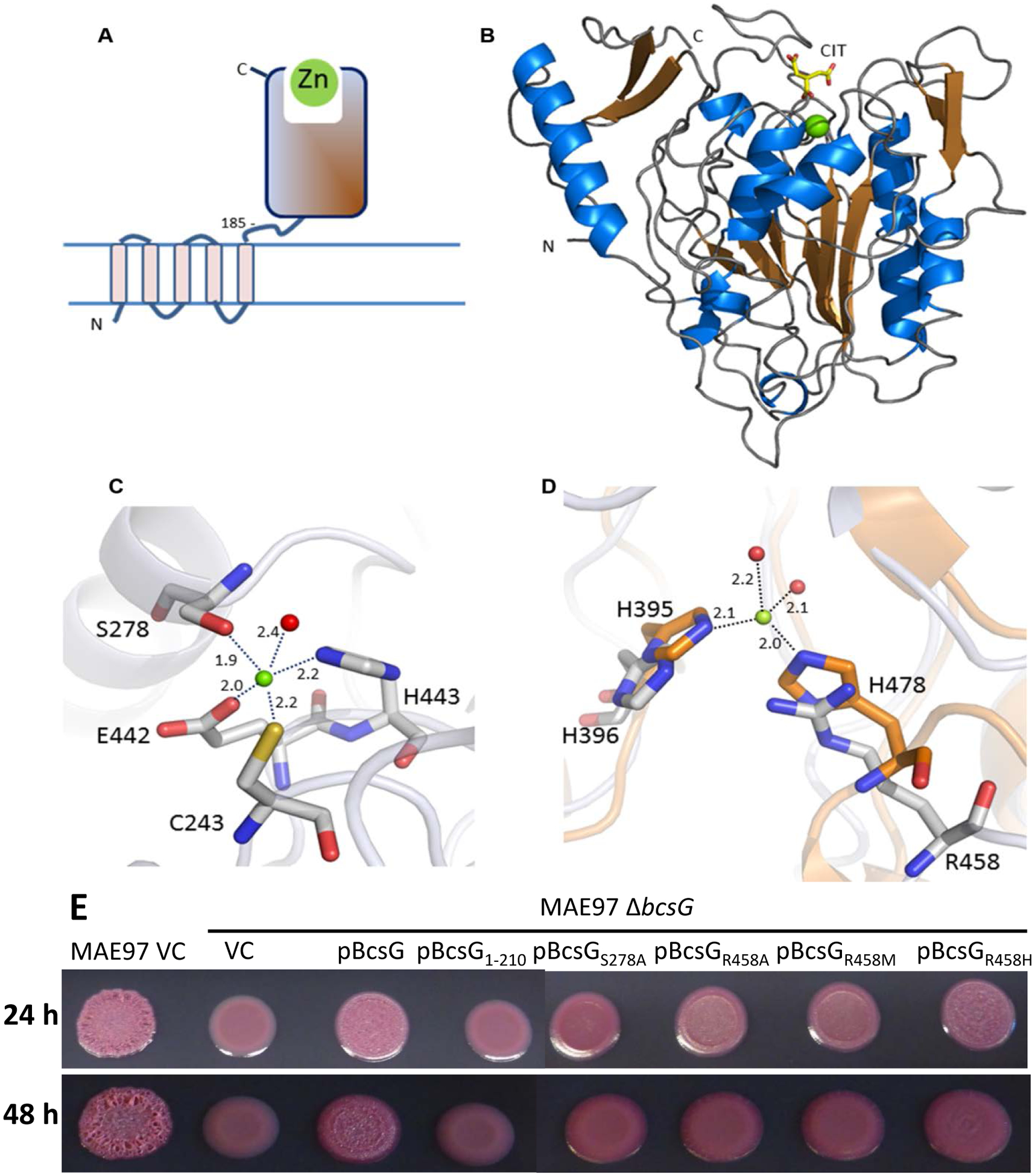
A. Domain structure of BcsG. The N-terminal transmembrane domain (residues 1–165) is linked to the C-terminal alkaline phosphatase-like domain (residues 185–559) via flexible inter-domain linker (residues 166–210). The fragment from aa 185 to 559 was used in the construct designed for crystallization. B. Schematic cartoon of the C-terminal alkaline phosphatase-type domain of BcsG. The zinc ion is shown as a green sphere and the citrate molecule bound close to the metal binding site as a stick-model. C. Metal binding site in BcsG. Distances between the Zn2+ and the coordinating atoms are indicated in Å. The water molecule is shown as a red sphere. D. View of the second Zn2+-binding site in the alkaline phosphatase family illustrated after superimposition of the phosphoethanolamine transferase MCR-2 (PDB code 5MX9) (orange carbon atoms) with BcsG (grey carbon atoms). While NmEptA contains a fully functional second Zn2+ site [35], one of the metal ligands, His478 in NmEptA, is replaced by Arg458 in BcsG, making binding of Zn2+ to this site less likely. E. Cellulose production in S. typhimurium strain MAE97 and its ΔbcsG mutants complemented with wild-type BcsG, the mutant lacking the alkaline phosphatase-type domain, and the mutants affecting the residues Ser278 and Arg458, shown in panels C and D. Other labels are as in Fig. 2. Cells were grown on salt-free LB agar plates for 24 h at 37 °C.
