Abstract
Background
The genetic-epigenetic theory postulates that endometriosis is triggered by a cumulative set of genetic-epigenetic (GE) incidents. Pelvic and upper genital tract infection might induce GE incidents and thus play a role in the pathogenesis of endometriosis. Thus, this article aims to review the association of endometriosis with upper genital tract and pelvic infections.
Methods
Pubmed, Scopus and Google Scholar were searched for ‘endometriosis AND (infection OR PID OR bacteria OR viruses OR microbiome OR microbiota)’, for ‘reproductive microbiome’ and for ‘reproductive microbiome AND endometriosis’, respectively. All 384 articles, the first 120 ‘best match’ articles in PubMed for ‘reproductive microbiome’ and the first 160 hits in Google Scholar for ‘reproductive microbiome AND endomytriosis’ were hand searched for data describing an association between endometriosis and bacterial, viral or other infections. All 31 articles found were included in this manuscript.
Results
Women with endometriosis have a significantly increased risk of lower genital tract infection, chronic endometritis, severe PID and surgical site infections after hysterectomy. They have more colony forming units of Gardnerella, Streptococcus, Enterococci and Escherichia coli in the endometrium. In the cervix Atopobium is absent, but Gardnerella, Streptococcus, Escherichia, Shigella, and Ureoplasma are increased. They have higher concentrations of Escherichia Coli and higher concentrations of bacterial endotoxins in menstrual blood. A Shigella/Escherichia dominant stool microbiome is more frequent. The peritoneal fluid of women with endometriosis contains higher concentrations of bacterial endotoxins and an increased incidence of mollicutes and of HPV viruses. Endometriosis lesions have a specific bacterial colonisation with more frequently mollicutes (54%) and both high and medium-risk HPV infections (11%). They contain DNA with 96% homology with Shigella. In mice transplanted endometrium changes the gut microbiome while the gut microbiome influences the growth of these endometriosis lesions.
Conclusions
Endometriosis is associated with more upper genital tract and peritoneal infections. These infections might be co-factors causing GE incidents and influencing endometriosis growth.
Keywords: Endometriosis, pathophysiology, infection, prevention of endometriosis, adolescent endometriosis
Introduction
Endometriosis is defined as ‘endometrium like cells outside the uterus’. Clinically endometriosis is a hereditary and heterogeneous disease with a variable presentation. It occurs in women without endometrium (Kawano et al., 2014) and in men taking estrogens (Rei et al., 2018) and even in those not taking estrogens (Giannarini et al., 2006; Jabr and Mani, 2014). Endometriosis is associated with pain, infertility, adenomyosis and altered pregnancy outcomes. Women with endometriosis have many biochemical changes in the endometrium, in plasma and in peritoneal fluid. The endometriosis lesions are clonal in origin and they have numerous and variable biochemical alterations such as aromatase activity and progesterone resistance. These observations can be explained by the genetic- epigenetic theory (Koninckx et al., 2019). The set of genetic and epigenetic changes inherited at birth (Simpson et al., 1980; Coxhead and Thomas, 1993; Moen, 1994; Hadfield et al., 1997; Kennedy, 1998; Kennedy et al., 1998; Treloar et al., 1999; Moen and Magnus, 1993) could explain the predisposition, the changes in the endometrium and in plasma (Bulun et al., 2015), the changes in immunology (Herington et al., 2011; PaulDmowski and Braun, 2004; Riccio et al., 2018; Zhang et al., 2018), the decreased defence mechanism against oxidative stress (Asghari et al., 2018) and the associated pregnancy disorders (Koninckx et al., 2018). When in some cells additional genetic or epigenetic incidents reach a certain threshold (Koninckx et al., 2019) the cells start to develop as endometriosis lesions. Further development of these lesions will vary with the specific set of genetic and epigenetic changes, and the environment of the peritoneal cavity or the ovary. This environment is different from the uterine environment by a different immunology, endocrinology, growth factors and cytokines. In addition, outside the uterus there is no junctional zone. Epigenetic and genetic changes can be caused by random errors during cell cleavage and by factors as radiation (Gordts et al., 2017), pollution with dioxins (Bruner-Tran and Osteen, 2010; Guo et al., 2009; Rier and Foster, 2002; Sofo et al., 2015), and oxidative stress (Augoulea et al., 2012; Gupta et al., 2006; Ito et al., 2017; Scutiero et al., 2017). Especially the oxidative stress of blood may be important in the uterine cavity, in the peritoneal cavity after retrograde menstruation (Donnez et al., 2016) and in the endometriosis lesions (Metzger et al., 1988). Also infection (Bierne et al., 2012; Ewald and Ewald, 2012) and viruses (Clarke and Clements, 1991; Perales et al., 2011; Akhter et al., 2014; Milavetz and Balakrishnan, 2015; Zhu et al., 2016) can induce genetic and epigenetic changes. Infection moreover increases oxidative stress and changes immune responses (Campos et al., 2018; Khan et al., 2018), and was suggested to be a cause of endometriosis (Khan et al., 2018).
The important roles of the microbiome of the gut and of the uterus and upper genital tract were only recently realised. The peritoneal cavity and the uterus are not sterile environments but contain specific microbial communities (Chen et al., 2017). The intra-uterine microbiome (Baker et al., 2018) affects embryo implantation as evidenced during IVF (de Ziegler et al., 2016; Moreno et al., 2016). Diet and lifestyle affect the microbiome of the gut (Conlon and Bird, 2014). Gut microbiota supply essential nutrients, synthetize vitamins, and play a role in angiogenesis and epithelial repair. Changes of the gut microbiota contribute to the development and progression of diseases, such as inflammatory bowel diseases, arthritis, psoriasis and cancer. Gut microbiota could influence the development of endometriosis through modulation of the immune responses and of pelvic inflammation (Laschke and Menger, 2016), by metabolising oestrogens (Baker, et al., 2017) and by affecting circulating oestrogen concentrations (Flores, et al., 2012).
The effect of sperm cells, which can attach and transport microbes and chlamydia on their tails (Wolner-Hanssen and Mardh, 1984; Confino et al., 1987; Friberg et al., 1987), on the upper genital tract and pelvic microbiome is poorly investigated. This mechanism of sperm transport of infectious agents was used to explain that the risk of non-gonococcal pelvic inflammatory disease (PID) (Toth, 1987) decreases when cervical mucus was less permeable to spermatozoa during the intake of combined oral contraceptives (Rosenberg, 1991). However, over the last decade this protective effect of combined oral contraceptives has become less clear. Changes in sexual behaviour as suggested by the increase in chlamydia infections (Datta et al., 2018) might affect the incidence of PID. Others suggested that the decrease in PID in women using oral contraceptives might result from fewer diagnoses because of less severe PID symptoms (Barrett and Taylor, 2005; Mitchell and Prabhu, 2013). Also the association of PID and ovarian cancer (Shen et al., 2016; Rasmussen et al., 2017; Zhou et al., 2017; Stewart et al., 2018; Trabert et al., 2018), and the decreased risk of ovarian cancer following tubal ligation (Cibula et al., 2011; Ness et al., 2011; Rice et al., 2012; Sieh et al., 2013; Ylikorkala, 2001) and following oral contraceptive use (Group, 2005; Ness et al., 2000) could be related PID. The unclear association of endometriosis and ovarian cancer (Guo, 2015; Ruderman and Pavone, 2017; Dawson et al., 2018; Muangtan et al., 2018) thus could have infection in common.
Considering the (epi)genetic theory of endometriosis (Koninckx, et al., 2019) and the observations that infections (Bierne et al., 2012) and viruses (Clarke and Clements, 1991; Perales et al., 2011; Akhter et al., 2014, Zhu et al., 2016), especially retroviruses (Kassiotis, 2014) are mutagenic with epigenetic effects (Milavetz and Balakrishnan, 2015) the fast growing literature that links endometriosis with upper genital tract and pelvic infections was reviewed.
Materials and Methods
We reviewed all published data on the association of human endometriosis and bacterial or viral infections till October 2019. Pubmed was searched for ‘endometriosis [Title] AND (infection OR PID OR bacteria OR viruses OR microbiome OR microbiota OR ‘pelvic inflammatory disease’ OR microbial). The title and if suggestive the pdf of all 384 articles found were hand searched by one author (P.R. Koninckx) for a link between endometriosis and infection. Pubmed was searched for ‘reproductive microbiome’ and the 120 best matched articles out of 1899 were hand searched. All articles of interest were added to a dedicated group in a personal endnote database. Google scholar was searched for ‘reproductive microbiome AND endometriosis’. The first 160 hits were searched for a relationship with endometriosis generating 8 additional articles of interest. A series of very recent non-peer reviewed articles on endometriosis treatment by antibiotics and food intake were not incorporated in this review.
The role of spermatozoa in PID was searched by (sperm OR spermatozoa) AND (PID OR pelvic infection) generating 176 hits and by (sperm OR spermatozoa) AND (flat capillaries OR cervical mucus) generating 1560 hits. Also, these articles were hand searched for evidence of the transport of infectious agents by spermatozoa.
All 31 articles describing a link between endometriosis and infection and or microbiota were incorporated in this manuscript. Since selection was done without eligibility criteria, and since all articles were included, a PRISMA flow chart was not included (Moher et al., 2009).
For clarity, throughout our manuscript we refer to microbiota as the group of microorganisms present in a defined environment, and microbiome refers to the entire habitat, thus including microorganisms, their genomes and the environmental conditions.
Results
Already in 1960 endometriosis was found in 18 to 50% of women with chronic salpingitis versus a small percent in women with acute infections (Jacobson, 1960).
Women with endometriosis have more upper genital tract infections
Indeed, a higher incidence of chronic endometritis (Cicinelli et al., 2017; Takebayashi et al., 2014), more severe pelvic inflammatory disease (Elizur et al., 2014), a higher risk of surgical site infection after hysterectomy (Chen et al., 2018) and a higher vaginal pH and more colony forming units of Gardnerella, a-Streptococcus, Enterococci and Escherichia coli in the endometrium (Khan et al., 2014). Women with cystic ovarian and deep endometriosis have in their cervix no Atopobium but an increase in Gardnerella, Streptococcus, Escherichia, Shigella, and Ureoplasma, and more frequently a Shigella/ Escherichia dominant stool microbiome (Ata et al., 2019). Women with endometriosis, especially with red lesions have a higher concentration of Escherichia Coli in menstrual blood and higher concentrations of bacterial endotoxins in menstrual blood and in peritoneal fluid (Khan et al., 2010; Khan et al., 2018). Women with endometriosis have a specific microbial colonisation in the uterus and in the peritoneal fluid (Chen et al., 2017) and in the uterus and fluid of cystic ovarian endometriosis (Khan et al., 2016). They have more frequently Enterobacteriaceae and Streptococcus in their cervical mucus (Akiyama et al., 2019). The significantly altered cervical microbiome in endometriosis stage III becomes normal for a short period after surgery (Cregger et al., 2017).
A history of PID is associated with a 3-fold increased risk of developing endometriosis within 10 years in comparison with control women (Tai et al., 2018). Chronic endometritis is associated with an altered uterine contractility which might increase retrograde menstruation (Pinto et al., 2015). Lower genital tract infection is an independent risk factor for developing endometriosis with and odds ratio of 2 in comparison with a control group (Lin et al., 2016).
Women with endometriosis have more mollicutes in peritoneal fluid
Mollicutes (mycoplasma hominis, mycoplasma genitalium, ureaplasma urealyticum, and ureaplasma parvum) were found in the peritoneal fluid of women with and without endometriosis in 54% and 33% respectively (Campos et al., 2018). Although not statistically significant when compared individually, the prevalences of mycoplasma hominis, mycoplasma genitalium, ureaplasma urealyticum were systematically higher in peritoneal fluid of women with endometriosis than in women without endometriosis. In peritoneal fluid of women with endometriosis, M. genitalium, M. hominis and U. urealyticum were found whereas in the control group only M. genitalium and M. hominis were detected. In the peritoneal biopsies of women with endometriosis M. genitalium and M. hominis were found whereas in the control group only M. genitalium was detected. Both peritoneal fluid and peritoneal biopsies showed a significant higher diversity in microorganisms in women with endometriosis than in the control group (Campos et al., 2018).
Endometriosis lesions contain frequently HPVA viruses
Although an initial report did not find increased viral DNA in endometriosis (Vestergaard et al., 2010), more recently high and medium risk human papilloma viruses (HPV), but not herpes virus or chlamydia were found in 11% of the endometriosis lesions and in 27% of other pelvic tissues (Oppelt et al., 2010). High-risk and medium-risk HPV were detected in 26% and 10% of ovaries with and without a cystic ovarian endometriosis (Heidarpour et al., 2017). In a prospective case control study, endometriosis was specifically associated with upper genital tract high risk HPV infection but not with other sexually transmitted diseases (Rocha et al., 2019).
Although the significance remains unclear, endometriosis lesions contain DNA with 96% homology with Shigella DNA (Kodati et al., 2008).
In mice, induced endometriosis interacts with the gut microbiome and vice-versa
In mice, induction of endometriosis altered the gut microbiota with an increased Firmicutes/ Bacteroidetes ratio (Yuan et al., 2018). In a mouse endometriosis model, the treatment with broad spectrum antibiotics or by metronidazole changed the intestinal microbiome and the growth of ‘endometriosis’ lesions was significantly reduced (Chadchan et al., 2019) unless gut microbiota were restored.
Discussion
The specific microbiome of the upper genital tract and of the peritoneal cavity
The peritoneal cavity, the upper genital tract, the endometrial cavity and the cervix have a specific microbiome which seems to be a continuum which is determined by the vaginal microbiome like ascending infections together with the specific local immunity. The microbiome of the peritoneal cavity is in addition influenced by the gut microbiome by transmural migration.
The role of the uterine microbiome in fertility and implantation (Moreno et al., 2016; de Ziegler et al., 2016; Baker et al., 2018; Liu et al., 2019) is beyond the scope of this article. The role of the microbiome of the peritoneal cavity and of the upper genital tract is unclear. This microbiome is considered a primary defence mechanism against infection by pathogens (Huang et al., 2014). The peritoneal microbiome plays a role in the innate immunity through bacterial endotoxins or lipopolysaccharides and the Toll-like receptor 4 (Khan et al., 2018).
The association of endometriosis with upper genital tract and peritoneal cavity infections
The association of endometriosis and infection started with Sampson who described histological similarities between endometriosis and infectious lesions (Sampson, 1927). Subtle endometriosis lesions have often a mainly inflammatory histology (Cabana et al., 2010; Marsh and Laufer, 2005). Ascending infections are consistent with the observation that endometriosis occurs predominantly in the pelvis, and were recently suggested to cause endometriosis by cell trauma (Canis et al., 2017). The apparent increase in severity of deep endometriosis in the western word (Koninckx et al., 2016), could be linked to the changed sexuality and the associated risk of infection. In addition our life style and increased hygiene (Cypers et al., 2014) were suggested to have induced changes in gut microbiome and auto-immunity and in enterococcal bacteriophages facilitating the transmural migration of gut bacteria (Duerkop et al., 2014).
The cumulative evidence that endometriosis is associated with more vaginal and upper genital tract infection and more PID (Elizur et al., 2014) is strong. Not a single report found an association in the opposite direction. The association of endometriosis with infections adds to the many endometriosis associated events (Koninckx et al., 2019). However, the interpretation of associated events should be done carefully, since for many associated events it is unclear which cause endometriosis, which are a consequence and which are the consequence of a common constitution.
Infections could be the consequence of any of the many endometriosis associated events such as infertility, pain, low grade pelvic inflammation (Halme, 1991; Samimi et al., 2019), more abundant menstruation, menorrhagia, alterations in immunology, changes in the endometrium and pregnancy disorders (Koninckx et al., 2019). In addition, medical therapy with e.g. progestagens will affect the vaginal epithelium and flora, the endometrium and endometrial microbiota and probably the peritoneal fluid.
Infections could be a co factor causing endometriosis
Bacteria (Bierne et al., 2012, Ewald and Ewald, 2012), viruses (Perales et al., 2011; Clarke and Clements, 1991; Akhter et al., 2014; Milavetz and Balakrishnan, 2015; Zhu et al., 2016) and other micro-organisms can cause genetic and epigenetic incidents in endometrial cells and in cells of the peritoneal cavity. Although not yet identified in the peritoneal cavity or in endometriosis lesions, it cannot be excluded that occasionally other viruses as retroviruses with a strong oncologic potential might find their way to the peritoneal cavity (Kassiotis, 2014). Besides a direct genetic or epigenetic effect, these infectious agents and the increased endotoxins increase the cellular and immunological stress and add to the oxidative stress of retrograde menstruation and of bleedings in the lesions (Kobayashi et al., 2014). Since tubal sterilisation decreases the risk of ovarian cancer, it is tempting to speculate that these infections could also cause tubal epithelial damage and ovarian cancer. Infection thus could be the common denominator to explain the unclear association of endometriosis and ovarian cancer.
Infections could contribute to the growth of endometriosis lesions through their effect on immunity, angiogenic and growth factors in the peritoneal cavity (Koninckx et al., 2016). Mycoplasma genitalium down-regulates genes associated with the inflammatory response (Campos et al., 2018). This together with the secretion of glycodelins (placental protein, PP14) by endometriotic cells (Okamoto et al., 1991) and the subsequent decrease in Natural Killer cell activity (Oosterlynck et al., 1991; Kanzaki et al., 1992) plays a role in the immuno-tolerance towards endometriosis. The stimulation of innate immunity by endotoxins plays a role in the low- grade inflammation of the peritoneal cavity in women with endometriosis (Halme, 1991; Khan et al., 2018; Samimi et al., 2019), which thus could be a co-factor in the growth of endometriosis lesions (Khan et al., 2018).
Endometriosis and intestinal microbiome
The role of the gut microbiota in endometriosis is still unclear. Transmural migration contributes to the peritoneal microbiome, and the finding of Shigella- like DNA in endometriosis lesions suggest a bowel origin (Kodati et al., 2008). With all limitations of the mouse model of endometriosis it is clear that gut microbiota affect the growth of transplanted endometrium (Chadchan et al., 2019; Yuan et al., 2018). It is unclear whether the mechanism involves the peritoneal microbiome, or associated growth factors. It explains observations in the human as the association of the endometriosis risk with diet as red meat (Yamamoto et al., 2018) or lipid intake or exercise since both diet (de Clercq et al., 2016) and exercise (Clark and Mach, 2016) affect gut microbiota.
Sperm transport of infectious agents and pelvic and upper genital microbiota
Spermatozoa can transport some bacteria on their tails (Confino et al., 1987). It is surprising that the transport capacity by spermatozoa remains unexplored for most bacteria and viruses and intra- cellular replicating pro-karyotic organisms. It is therefore also unknown whether sperm transport plays a role in tubal damage by chlamydia, or in the pathogenesis of endometriosis.
Upper genital tract and peritoneal cavity microbiome by sperm transport of bacteria obviously are at best only one factor in the pathophysiology of endometriosis since the disease exists in the absence of sexual contact.
Strength and weakness of these studies
The weakness of the association of endometriosis with upper genital tract and pelvic infections is that the articles are either microbiota studies, or population-based association studies, or studies investigating a specific infection. In most studies the diagnosis and type of endometriosis is poorly described. Except for studies describing severe deep or cystic ovarian endometriosis, it is rarely clear how subtle endometriosis were classified. Recognition of subtle lesions varies with the expertise and interest of the surgeon and it remains debated whether subtle endometriosis is pathology or whether it a physiological phenomenon occurring intermittently in all women (Koninckx et al., 2019). However, this does not invalidate the conclusion. On the contrary a stricter definition of endometriosis is expected to strengthen the conclusions of an association between endometriotic disease and infection.
A publication bias is likely since studies with a negative outcome risk are less likely to be published.
Studies on PID and of the natural defence mechanisms are hampered by the inaccurate clinical diagnosis of PID, except for gonorrhoea with severe symptoms. An accurate diagnosis of PID requires a diagnostic laparoscopy and the identification of the infectious agents, which requires advanced laboratory techniques for some viruses and some bacteria. In addition, PID is often a poly-viral and/ or poly-bacterial infection.
Conclusions
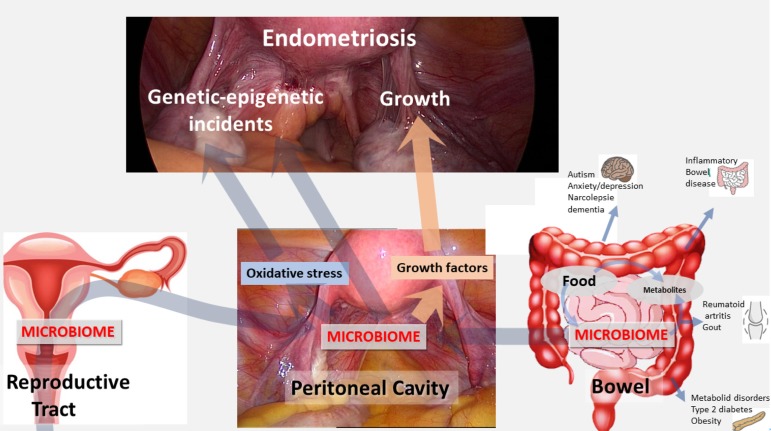
In conclusion, the uterine, tubal and pelvic cavities are not sterile, but they are environments with a specific immunity and microbiome. This is not surprising considering the connection to the outside world and the potential transport of micro- organisms by spermatozoa. The contribution of transmural migration of the gut microbiota is unclear. Endometriosis is associated with alterations in the microbiome of the upper-genital tract and the peritoneal cavity, with an increase in mollicutes and with HPV virus in the lesions. These infections have the potential to initiate endometriosis by causing genetic-epigenetic incidents and to contribute to the growth of endometriosis (Figure 1). Understanding the mechanism of upper genital tract infection and microbiota and the interplay with the gut microbiota is expected to lead to new therapies for prevention of endometriosis growth and recurrences.
Figure 1.
The peritoneal microbiome results from the uterine and upper-genital tract microbiome and the gut microbiome. The peritoneal microbiome can cause endometriosis by inducing genetic epigenetic incidents either directly or by increasing the oxidative stress. The peritoneal microbiome also can increase endometriosis growth through growth factors and immunologic changes. This explains why the gut microbiome, which is influenced by food intake and exercise, can influence the induction and growth of endometriosis, besides many other effects.
References
- 1.Akhter J, Ali Aziz MA, Al Ajlan A, et al. Breast cancer: is there a viral connection? Adv Anat Pathol. 2014;21:373–381. doi: 10.1097/PAP.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama K, Nishioka K, Khan KN, et al. Molecular detection of microbial colonization in cervical mucus of women with and without endometriosis. Am J Reprod Immunol. 2019:e13147. doi: 10.1111/aji.13147. [DOI] [PubMed] [Google Scholar]
- 3.Asghari S, Valizadeh A, Aghebati-Maleki L, et al. Endometriosis: Perspective, lights, and shadows of etiology. Biomed Pharmacother. 2018;106:163–174. doi: 10.1016/j.biopha.2018.06.109. [DOI] [PubMed] [Google Scholar]
- 4.Ata B, Yildiz S, Turkgeldi E, et al. The Endobiota Study: Comparison of Vaginal, Cervical and Gut Microbiota Between Women with Stage 3/4 Endometriosis and Healthy Controls. Sci Rep. 2019;9:2204. doi: 10.1038/s41598-019-39700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Augoulea A, Alexandrou A, Creatsa M, et al. Pathogenesis of endometriosis: the role of genetics, inflammation and oxidative stress. Arch Gynecol Obstet. 2012;286:99–103. doi: 10.1007/s00404-012-2357-8. [DOI] [PubMed] [Google Scholar]
- 6.Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas. 2017;103:45–53. doi: 10.1016/j.maturitas.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 7.Baker JM, Chase DM, Herbst-Kralovetz MM. Front Immunol. Uterine Microbiota: Residents, Tourists, or Invaders. 2018;9:208. doi: 10.3389/fimmu.2018.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrett S, Taylor C. A review on pelvic inflammatory disease. International journal of STD & AIDS. 2005;16:715–720. doi: 10.1258/095646205774763270. [DOI] [PubMed] [Google Scholar]
- 9.Bierne H, Hamon M, Cossart P. Epigenetics and bacterial infections. Cold Spring Harb Perspect Med. 2012;2:a010272. doi: 10.1101/cshperspect.a010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruner-Tran KL, Osteen KG. Dioxin-like PCBs and endometriosis. Syst Biol Reprod Med. 2010;56:132–146. doi: 10.3109/19396360903381023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulun SE, Monsivais D, Kakinuma T, et al. Molecular biology of endometriosis: from aromatase to genomic abnormalities. Semin Reprod Med. 2015;33:220–224. doi: 10.1055/s-0035-1554053. [DOI] [PubMed] [Google Scholar]
- 12.Cabana MD, Forster-Barber AE, Hong T, et al. Teen troubled by a trembling leg. Contemporary Pediatrics. 2010;27:22–27. [Google Scholar]
- 13.Campos GB, Marques LM, Rezende IS, et al. Mycoplasma genitalium can modulate the local immune response in patients with endometriosis. Fertil Steril. 2018;109:549–560. doi: 10.1016/j.fertnstert.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Canis M, Bourdel N, Houlle C, et al. Trauma and endometriosis. A review. May we explain surgical phenotypes and natural history of the disease? J Gynecol Obstet Hum Reprod. 2017;46:219–227. doi: 10.1016/j.jogoh.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Chadchan SB, Cheng M, Parnell LA, et al. Antibiotic therapy with metronidazole reduces endometriosis disease progression in mice: a potential role for gut microbiota. Hum Reprod. 2019;34:1106–1116. doi: 10.1093/humrep/dez041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C, Song X, Wei W, et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun. 2017;8:875. doi: 10.1038/s41467-017-00901-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen I, Choudhry AJ, Schramm D, et al. Type of Pelvic Disease as a Risk Factor for Surgical Site Infectionin Women Undergoing Hysterectomy. J Minim Invasive Gynecol. 2018;26:1149–1156. doi: 10.1016/j.jmig.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Cibula D, Widschwendter M, Majek O, et al. Tubal ligation and the risk of ovarian cancer: review and meta-analysis. Hum Reprod Update. 2011;17:55–67. doi: 10.1093/humupd/dmq030. [DOI] [PubMed] [Google Scholar]
- 19.Cicinelli E, Trojano G, Mastromauro M, et al. Higher prevalence of chronic endometritis in women with endometriosis: a possible etiopathogenetic link. Fertil Steril. 2017;108:289–295.:e281. doi: 10.1016/j.fertnstert.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Clark A, Mach N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: a systematic review for athletes. J Int Soc Sports Nutr. 2016;13:43. doi: 10.1186/s12970-016-0155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke P, Clements JB. Mutagenesis occurring following infection with herpes simplex virus does not require virus replication. Virology. 1991;182:597–606. doi: 10.1016/0042-6822(91)90600-g. [DOI] [PubMed] [Google Scholar]
- 22.Confino E, Friberg J, Silverman S, et al. Penetration of bacteria and spermatozoa into bovine cervical mucus. Obstet Gynecol. 1987;70:134–136. [PubMed] [Google Scholar]
- 23.Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2014;7:17–44. doi: 10.3390/nu7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coxhead D, Thomas EJ. Familial inheritance of endometriosis in a British population. A case control study. J Obstet Gynecol. 1993;13:42–44. [Google Scholar]
- 25.Cregger MA, Lenz K, Leary E, et al. Reproductive Microbiomes: Using the Microbiome as a Novel Diagnostic Tool for Endometriosis. Reproductive Immunology: Open Access. 2017;2:36. [Google Scholar]
- 26.Cypers H, Van Praet L, Varkas G, et al. Relevance of the gut/joint axis for the management of spondyloarthritis in daily clinical practice. Curr Opin Rheumatol. 2014;26:371–376. doi: 10.1097/BOR.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 27.Datta S, Mercer CH, Keeling MJ. Capturing sexual contact patterns in modelling the spread of sexually transmitted infections: Evidence using Natsal-3. PLoS One. 2018;13:e0206501. doi: 10.1371/journal.pone.0206501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dawson A, Fernandez ML, Anglesio M, et al. Endometriosis and endometriosis-associated cancers: new insights into the molecular mechanisms of ovarian cancer development. Ecancermedicalscience. 2018;12:803. doi: 10.3332/ecancer.2018.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Clercq NC, Groen AK, Romijn JA, et al. Gut Microbiota in Obesity and Undernutrition. Adv Nutr. 2016;15(7):1080–1089. doi: 10.3945/an.116.012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Ziegler D, Pirtea P, Galliano D, et al. Optimal uterine anatomy and physiology necessary for normal implantation and placentation. Fertil Steril. 2016;105:844–854. doi: 10.1016/j.fertnstert.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 31.Donnez J, Binda MM, Donnez O, et al. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil Steril. 2016;106:1011–1017. doi: 10.1016/j.fertnstert.2016.07.1075. [DOI] [PubMed] [Google Scholar]
- 32.Duerkop BA, Palmer KL, Horsburgh MJ. Enterococcal Bacteriophages and Genome Defense. Internet. [PubMed] [Google Scholar]
- 33. Gilmore MS, Clewell DB. Enterococci: From Commensals to Leading Causes of Drug Resistant Infection. Ike Y and Shankar N (eds) 2014. Massachusetts Eye and Ear Infirmary; , Boston: [PubMed] [Google Scholar]
- 34.Elizur SE, Lebovitz O, Weintraub AY, et al. Pelvic inflammatory disease in women with endometriosis is more severe than in those without. Aust N Z J Obstet Gynaecol. 2014;54:162–165. doi: 10.1111/ajo.12189. [DOI] [PubMed] [Google Scholar]
- 35.Ewald PW, Swain Ewald HA. Infection, mutation, and cancer evolution. J Mol Med. 2012;90:535–541. doi: 10.1007/s00109-012-0891-2. [DOI] [PubMed] [Google Scholar]
- 36.Flores R, Shi J, Fuhrman B, et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med. 2012;21(10):253. doi: 10.1186/1479-5876-10-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friberg J, Confino E, Suarez M, et al. Chlamydia trachomatis attached to spermatozoa recovered from the peritoneal cavity of patients with salpingitis. J Reprod Med. 1987;32:120–122. [PubMed] [Google Scholar]
- 38.Giannarini G, Scott CA, Moro U, et al. Cystic endometriosis of the epididymis. Urology. 2006;68:203–203. doi: 10.1016/j.urology.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 39.Gordts S, Koninckx P, Brosens I. Pathogenesis of deep endometriosis. Fertil Steril. 2017;108:872–885. doi: 10.1016/j.fertnstert.2017.08.036. [DOI] [PubMed] [Google Scholar]
- 40. ECW Group. Noncontraceptive health benefits of combined oral contraception. Hum Reprod Update. 2005;11:513–525. doi: 10.1093/humupd/dmi019. [DOI] [PubMed] [Google Scholar]
- 41.Guo SW. Endometriosis and ovarian cancer: potential benefits and harms of screening and risk-reducing surgery. Fertil Steril. 2015;104:813–830. doi: 10.1016/j.fertnstert.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Guo SW, Simsa P, Kyama CM, et al. Reassessing the evidence for the link between dioxin and endometriosis: from molecular biology to clinical epidemiology. Mol Hum Reprod. 2009;15:609–624. doi: 10.1093/molehr/gap075. [DOI] [PubMed] [Google Scholar]
- 43.Gupta S, Agarwal A, Krajcir N, et al. Role of oxidative stress in endometriosis. Reprod Biomed Online. 2006;13:126–134. doi: 10.1016/s1472-6483(10)62026-3. [DOI] [PubMed] [Google Scholar]
- 44.Hadfield RM, Mardon HJ, Barlow DH, et al. Endometriosis in monozygotic twins. Fertil Steril. 1997;68:941–942. doi: 10.1016/s0015-0282(97)00359-2. [DOI] [PubMed] [Google Scholar]
- 45.Halme J. Role of peritoneal inflammation in endometriosis-associated infertility. Ann N Y Acad Sci. 1991;622:266–274. doi: 10.1111/j.1749-6632.1991.tb37870.x. [DOI] [PubMed] [Google Scholar]
- 46.Heidarpour M, Derakhshan M, Derakhshan-Horeh M, et al. Prevalence of high-risk human papillomavirus infection in women with ovarian endometriosis. J Obstet Gynaecol Res. 2017;43:135–139. doi: 10.1111/jog.13188. [DOI] [PubMed] [Google Scholar]
- 47.Herington JL, Bruner-Tran KL, Lucas JA, et al. Immune interactions in endometriosis. Expert Rev Clin Immunol. 2011;7:611–626. doi: 10.1586/eci.11.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang B, Fettweis JM, Brooks JP, et al. The changing landscape of the vaginal microbiome. Clin Lab Med. 2014;34:747–761. doi: 10.1016/j.cll.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ito F, Yamada Y, Shigemitsu A, et al. Role of Oxidative Stress in Epigenetic Modification in Endometriosis. Reprod Sci. 2017;11:1493–1502. doi: 10.1177/1933719117704909. [DOI] [PubMed] [Google Scholar]
- 50.Jabr FI, Mani V. An unusual cause of abdominal pain in a male patient: Endometriosis. Avicenna J Med. 2014;4:99–101. doi: 10.4103/2231-0770.140660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacobson L. Infected pelvic endometriosis. Nord med. 1960;64:844–847. [PubMed] [Google Scholar]
- 52.Kanzaki H, Wang HS, Kariya M, et al. Suppression of natural killer cell activity by sera from patients with endometriosis. Am J Obstet Gynecol. 1992;167:257–261. doi: 10.1016/s0002-9378(11)91670-6. [DOI] [PubMed] [Google Scholar]
- 53.Kassiotis G. Endogenous retroviruses and the development of cancer. J Immunol. 2014;192:1343–1349. doi: 10.4049/jimmunol.1302972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawano Y, Hirakawa T, Nishida M, et al. Functioning endometrium and endometrioma in a patient with mayer-rokitanski-kuster-hauser syndrome. Jpn Clin Med. 2014;5:43–45. doi: 10.4137/JCM.S12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kennedy S. The genetics of endometriosis. Eur J Obstet Gynecol Reprod Biol. 1999;82:129–133. doi: 10.1016/s0301-2115(98)00213-9. [DOI] [PubMed] [Google Scholar]
- 56.Kennedy S, Hadfield R, Westbrook C, et al. Magnetic resonance imaging to assess familial risk in relatives of women with endometriosis. Lancet. 1998;352:1440–1441. doi: 10.1016/S0140-6736(05)61262-7. [DOI] [PubMed] [Google Scholar]
- 57.Khan KN, Fujishita A, Hiraki K, et al. Bacterial contamination hypothesis: a new concept in endometriosis. Reprod Med Biol. 2018;17:125–133. doi: 10.1002/rmb2.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khan KN, Fujishita A, Kitajima M, et al. Intra-uterine microbial colonization and occurrence of endometritis in women with endometriosisdagger. Hum Reprod. 2014;29:2446–2456. doi: 10.1093/humrep/deu222. [DOI] [PubMed] [Google Scholar]
- 59.Khan KN, Fujishita A, Masumoto H, et al. Molecular detection of intrauterine microbial colonization in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2016;199:69–75. doi: 10.1016/j.ejogrb.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 60.Khan KN, Kitajima M, Hiraki K, et al. Escherichia coli contamination of menstrual blood and effect of bacterial endotoxin on endometriosis. Fertil Steril. 2010;94:2860–2863.:e2861-2863. doi: 10.1016/j.fertnstert.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 61.Kobayashi H, Higashiura Y, Shigetomi H, et al. Pathogenesis of endometriosis: the role of initial infection and subsequent sterile inflammation. Mol Med Rep. 2014;9:9–15. doi: 10.3892/mmr.2013.1755. [DOI] [PubMed] [Google Scholar]
- 62.Kodati VL, Govindan S, Movva S, et al. Role of Shigella infection in endometriosis: a novel hypothesis. Med Hypotheses. 2008;70:239–243. doi: 10.1016/j.mehy.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 63.Koninckx PR, Gomel V, Ussia A, et al. Role of the peritoneal cavity in the prevention of postoperative adhesions, pain, and fatigue. Fertil Steril. 2016a;106:998–1010. doi: 10.1016/j.fertnstert.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 64.Koninckx PR, Ussia A, Adamyan L, et al. Pathogenesis of endometriosis: the genetic/epigenetic theory. Fertil Steril. 2019;111:327–340. doi: 10.1016/j.fertnstert.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 65.Koninckx PR, Ussia A, Keckstein J, et al. Epidemiology of subtle, typical, cystic, and deep endometriosis: a systematic review. Gynecol Surg. 2016b;13:457–467. [Google Scholar]
- 66.Koninckx PR, Zupi E, Martin DC. Endometriosis and pregnancy outcome. Fertil Steril. 2018;110:406–407. doi: 10.1016/j.fertnstert.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 67.Laschke MW, Menger MD. The gut microbiota: a puppet master in the pathogenesis of endometriosis? Am J Obstet Gynecol. 2016 e61-64;215(68):e61-64. doi: 10.1016/j.ajog.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 68.Lin WC, Chang CY, Hsu YA, et al. Increased Risk of Endometriosis in Patients With Lower Genital Tract Infection: A Nationwide Cohort Study. Medicine (Baltimore) 2016;95:e2773. doi: 10.1097/MD.0000000000002773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Y, Ko EY, Wong KK, et al. Endometrial microbiota in infertile women with and without chronic endometritis as diagnosed using a quantitative and reference range-based method. Fertil Steril. 2019;112:707–717. doi: 10.1016/j.fertnstert.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 70.Marsh EE, Laufer MR. Endometriosis in premenarcheal girls who do not have an associated obstructive anomaly. Fertil Steril. 2005;83:758–760. doi: 10.1016/j.fertnstert.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 71.Metzger DA, Olive DL, Haney AF. Limited hormonal responsiveness of ectopic endometrium: Histologic correlation with intrauterine endometrium. Hum Pathol. 1988;19:1417–1424. doi: 10.1016/s0046-8177(88)80234-x. [DOI] [PubMed] [Google Scholar]
- 72.Milavetz BI, Balakrishnan L. Viral epigenetics. Methods in molecular biology (Clifton, NJ) 2015;1238:569–596. doi: 10.1007/978-1-4939-1804-1_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mitchell C, Prabhu M. Pelvic inflammatory disease: current concepts in pathogenesis, diagnosis and treatment. Infect Dis Clin North Am. 2013;27:793–809. doi: 10.1016/j.idc.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moen MH. Endometriosis in monozygotic twins. Acta Obstet Gynecol Scand. 1994;73:59–62. doi: 10.3109/00016349409013396. [DOI] [PubMed] [Google Scholar]
- 75.Moen MH, Magnus P. The familial risk of endometriosis. Acta Obstet Gynecol Scand. 1993;72:560–564. doi: 10.3109/00016349309058164. [DOI] [PubMed] [Google Scholar]
- 76.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;21(6):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moreno I, Codoner FM, Vilella F, et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gynecol. 2016;215:684–703. doi: 10.1016/j.ajog.2016.09.075. [DOI] [PubMed] [Google Scholar]
- 78.Muangtan S, Suknikhom W, Sananpanichkul P. Epithelial Ovarian Cancer with Endometriosis is not Associated with Menopausal Status: a Co-Association Study at Prapokklao Hospital. Asian Pac J Cancer Prev. 2018;19:1337–1341. doi: 10.22034/APJCP.2018.19.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ness RB, Dodge RC, Edwards RP, et al. Contraception methods, beyond oral contraceptives and tubal ligation, and risk of ovarian cancer. Ann Epidemiol. 2011;21:188–196. doi: 10.1016/j.annepidem.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ness RB, Grisso JA, Cottreau C, et al. Factors related to inflammation of the ovarian epithelium and risk of ovarian cancer. Epidemiology. 2000;11:111–117. doi: 10.1097/00001648-200003000-00006. [DOI] [PubMed] [Google Scholar]
- 81.Okamoto N, Uchida A, Takakura K, et al. Suppression by human placental protein 14 of natural killer cell activity. Am J Reprod Immunol. 1991;26:137–142. doi: 10.1111/j.1600-0897.1991.tb00713.x. [DOI] [PubMed] [Google Scholar]
- 82.Oosterlynck DJ, Cornillie FJ, Waer M, et al. Women with endometriosis show a defect in natural killer activity resulting in a decreased cytotoxicity to autologous endometrium. Fertil Steril. 1991;56:45–51. doi: 10.1016/s0015-0282(16)54414-8. [DOI] [PubMed] [Google Scholar]
- 83.Oppelt P, Renner SP, Strick R, et al. Correlation of high-risk human papilloma viruses but not of herpes viruses or Chlamydia trachomatis with endometriosis lesions. Fertil Steril. 2010;93:1778–1786. doi: 10.1016/j.fertnstert.2008.12.061. [DOI] [PubMed] [Google Scholar]
- 84.Paul Dmowski W, Braun DP. Immunology of endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18:245–263. doi: 10.1016/j.bpobgyn.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 85.Perales C, Agudo R, Manrubia SC, et al. Influence of mutagenesis and viral load on the sustained low-level replication of an RNA virus. J Mol Biol. 2011;407:60–78. doi: 10.1016/j.jmb.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 86.Pinto V, Matteo M, Tinelli R, et al. Altered uterine contractility in women with chronic endometritis. Fertil Steril. 2015;103:1049–1052. doi: 10.1016/j.fertnstert.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 87.Rasmussen CB, Kjaer SK, Albieri V, et al. Pelvic Inflammatory Disease and the Risk of Ovarian Cancer and Borderline Ovarian Tumors: A Pooled Analysis of 13 Case-Control Studies. Am J Epidemiol. 2017;185:8–20. doi: 10.1093/aje/kww161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rei C, Williams T, Feloney M. Endometriosis in a Man as a Rare Source of Abdominal Pain: A Case Report and Review of the Literature. Case Rep Obstet Gynecol. 2018;2018:2083121. doi: 10.1155/2018/2083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Riccio L, Santulli P, Marcellin L, et al. Immunology of endometriosis. Best Pract Res Clin Obstet Gynaecol. 2018;50:39–49. doi: 10.1016/j.bpobgyn.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 90.Rice MS, Murphy MA, Tworoger SS. Tubal ligation, hysterectomy and ovarian cancer: A meta-analysis. J Ovarian Res. 2012;5:13. doi: 10.1186/1757-2215-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rier S, Foster WG. Environmental dioxins and endometriosis. Toxicol Sci. 2002;70:161–170. doi: 10.1093/toxsci/70.2.161. [DOI] [PubMed] [Google Scholar]
- 92.Rocha RM, Souza RP, Gimenes F, et al. The high-risk human papillomavirus continuum along the female reproductive tract and its relationship to infertility and endometriosis. Reprod Biomed Online. 2019;38:926–937. doi: 10.1016/j.rbmo.2018.11.032. [DOI] [PubMed] [Google Scholar]
- 93.Rosenberg M. Contraception and STDs. IPPF Med Bull. 1991;25:3–4. [PubMed] [Google Scholar]
- 94.Ruderman R, Pavone ME. Ovarian cancer in endometriosis: an update on the clinical and molecular aspects. Minerva Ginecol. 2017;69:286–294. doi: 10.23736/S0026-4784.17.04042-4. [DOI] [PubMed] [Google Scholar]
- 95.Samimi M, Pourhanifeh MH, Mehdizadehkashi A, et al. The role of inflammation, oxidative stress, angiogenesis, and apoptosis in the pathophysiology of endometriosis: Basic science and new insights based on gene expression. 2019;234:19384–19392. doi: 10.1002/jcp.28666. [DOI] [PubMed] [Google Scholar]
- 96.Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–469. [Google Scholar]
- 97.Scutiero G, Iannone P, Bernardi G, et al. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxid Med Cell Longev. 2017;2017:7265238. doi: 10.1155/2017/7265238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shen CC, Hu LY, Yang AC, et al. Risk of uterine, ovarian and breast cancer following pelvic inflammatory disease: a nationwide population-based retrospective cohort study. BMC cancer. 2016;16:839. doi: 10.1186/s12885-016-2857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sieh W, Salvador S, McGuire V, et al. Tubal ligation and risk of ovarian cancer subtypes: a pooled analysis of case-control studies. Int J Epidemiol. 2013;42:579–589. doi: 10.1093/ije/dyt042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Simpson JL, Elias S, Malinak LR, et al. Heritable aspects of endometriosis. I. Genetic studies. Am J Obstet Gynecol. 1980;137:327–331. doi: 10.1016/0002-9378(80)90917-5. [DOI] [PubMed] [Google Scholar]
- 101.Sofo V, Gotte M, Lagana AS, et al. Correlation between dioxin and endometriosis: an epigenetic route to unravel the pathogenesis of the disease. Arch Gynecol Obstet. 2015;292:973–986. doi: 10.1007/s00404-015-3739-5. [DOI] [PubMed] [Google Scholar]
- 102.Stewart LM, Spilsbury K, Jordan S, et al. Risk of high-grade serous ovarian cancer associated with pelvic inflammatory disease, parity and breast cancer. Cancer Epidemiol. 2018;55:110–116. doi: 10.1016/j.canep.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 103.Tai FW, Chang CY, Chiang JH, et al. Association of Pelvic Inflammatory Disease with Risk of Endometriosis: A Nationwide Cohort Study Involving 141,460 Individuals. J Clin Med. 2018;7:379. doi: 10.3390/jcm7110379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Takebayashi A, Kimura F, Kishi Y, et al. The association between endometriosis and chronic endometritis. PLoS One. 2014;9:e88354. doi: 10.1371/journal.pone.0088354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Toth A. The role of spermatozoa in the development of pelvic inflammatory disease in the woman. Advances in contraception : the official journal of the Society for the Advancement of Contraception. 1987;3:97–102. doi: 10.1007/BF01890697. [DOI] [PubMed] [Google Scholar]
- 106.Trabert B, Waterboer T, Idahl A, et al. Antibodies Against Chlamydia trachomatis and Ovarian Cancer Risk in Two Independent Populations. J Natl Cancer Inst. 2018;111:129–136. doi: 10.1093/jnci/djy084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Treloar SA, O’Connor DT, O’Connor VM, et al. Genetic influences on endometriosis in an Australian twin sample. Fertil Steril. 1999;71:701–710. doi: 10.1016/s0015-0282(98)00540-8. [DOI] [PubMed] [Google Scholar]
- 108.Vestergaard AL, Knudsen UB, Munk T, et al. Low prevalence of DNA viruses in the human endometrium and endometriosis. Arch Virol. 2010;155:695–703. doi: 10.1007/s00705-010-0643-y. [DOI] [PubMed] [Google Scholar]
- 109.Wolner-Hanssen P, Mardh PA. In vitro tests of the adherence of Chlamydia trachomatis to human spermatozoa. Fertil Steril. 1984;42:102–107. doi: 10.1016/s0015-0282(16)47966-5. [DOI] [PubMed] [Google Scholar]
- 110.Yamamoto A, Harris HR, Vitonis AF, et al. A prospective cohort study of meat and fish consumption and endometriosis risk. Am J Obstet Gynecol. 2018;219:178.:e171-178.e110. doi: 10.1016/j.ajog.2018.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ylikorkala O. Tubal ligation reduces the risk of ovarian cancer. Acta Obstet Gynecol Scand. 2001;80:875–877. doi: 10.1034/j.1600-0412.2001.801001.x. [DOI] [PubMed] [Google Scholar]
- 112.Yuan M, Li D, Zhang Z, et al. Endometriosis induces gut microbiota alterations in mice. Hum Reprod. 2018;33:607–616. doi: 10.1093/humrep/dex372. [DOI] [PubMed] [Google Scholar]
- 113.Zhang T, De Carolis C, Man GCW, et al. The link between immunity, autoimmunity and endometriosis: a literature update. Autoimmun Rev. 2018;17:945–955. doi: 10.1016/j.autrev.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 114.Zhou Z, Zeng F, Yuan J, et al. Pelvic inflammatory disease and the risk of ovarian cancer: a meta-analysis. Cancer Causes Control. 2017;28:415–428. doi: 10.1007/s10552-017-0873-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhu Y, Ma M, Huang J, et al. Effects of Hepatitis C Virus Infection on Human Sperm Chromosomes. Clin Lab. 2016;62:373–379. doi: 10.7754/clin.lab.2015.150705. [DOI] [PubMed] [Google Scholar]