Figure 4.
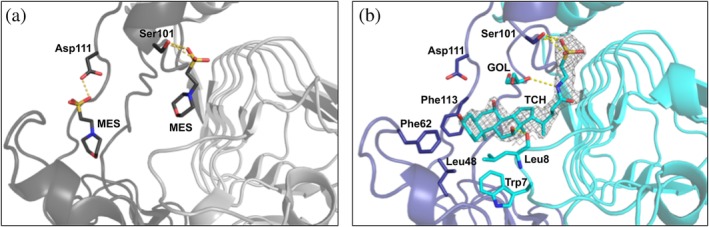
AfCAT structures in the presence of MES and taurocholate. (a) Zoomed view of the structure of AfCAT (PDB ID: 5UX9) in complex with two molecules of MES buffer. The acceptor site is located between two monomers, and residues of the AfCAT protein that form H‐bonds with MES buffer molecules are shown in dark gray sticks. H‐bonds are indicated with yellow dashed lines. The ribbon diagrams of two monomers are shown in dark gray and light gray, respectively. (b) AfCAT‐TCH (PDB ID: 6PXA) structure in complex with taurocholate (TCH) and glycerol (GOL). Ribbon diagrams of two monomers of the trimer are shown in purple and cyan, respectively. Key residues that line the acceptor site of the AfCAT structure are represented as sticks and colored according to the monomer in which they lie. H‐bonds between TCH, GOL, and the AfCAT protein are indicated with yellow dashed lines. The weighted 2F o–F c map in gray mesh surrounds the taurocholate molecule and is drawn at a contour level of 1σ. The secondary structures of each crystal structure were defined using Stride (http://webclu.bio.wzw.tum.de/stride/) and altered manually in PyMOL. All figures were prepared using PyMOL and Microsoft PowerPoint
