Figure 2 |. Mechanism of Fe-S cluster binding to the ANCL mutants of CSPα in neurons.
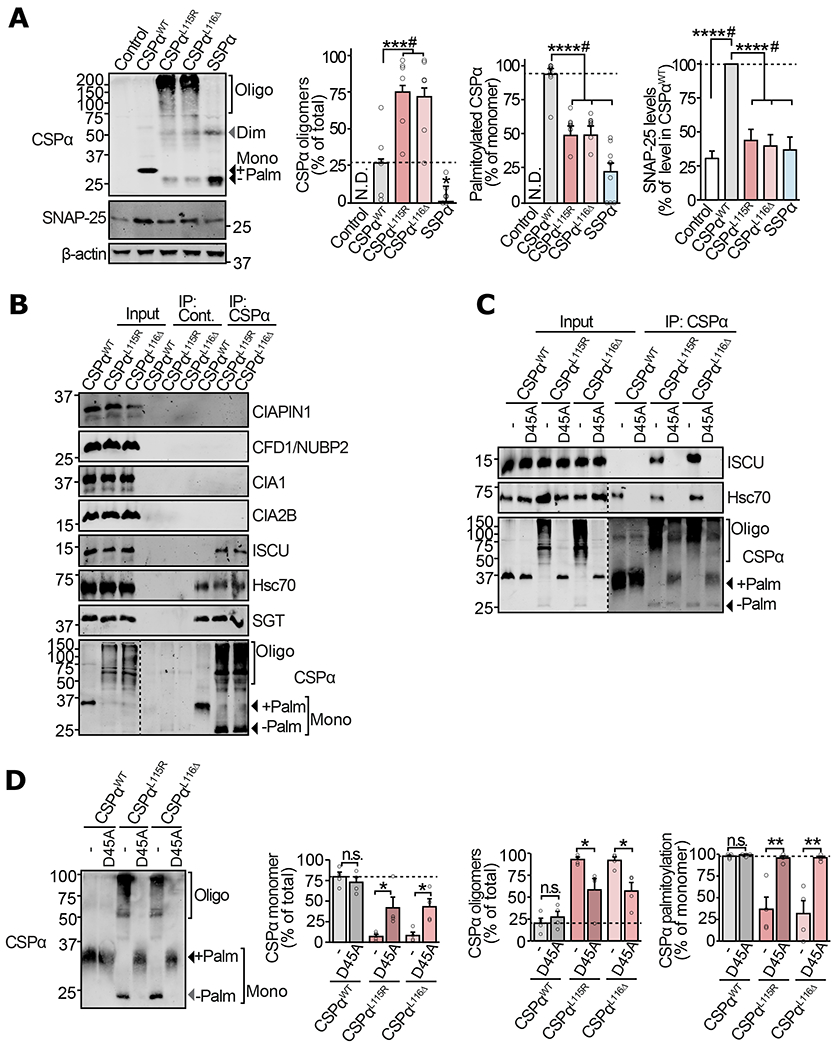
(a-d) Primary cortical neurons from neonatal CSPα−/− mice were infected with lentiviruses expressing the indicated myc-tagged version of CSPα on 7 days in vitro (DIV). (a) Neurons expressing CSPαWT, CSPαL115R, CSPαL116Δ, SSPα, or empty virus (control) were lysed on 17 DIV and CSPα oligomerization, palmitoylation, and SNAP-25 levels were measured by quantitative immunoblotting. SNAP-25 was normalized to β-actin and is shown as percent of its expression in CSPαWT expressing neurons. (b) Neurons expressing CSPαWT, CSPαL115R, or CSPαL116Δ were lysed on DIV14 and subjected to anti-myc immunoprecipitation. The eluate was immunoblotted to detect co-immunoprecipitated members of the CIA machinery. Hsc70 and SGT are known interactors of CSPα. (c) Neurons expressing CSPαWT, CSPαWT/D45A, CSPαL115R, CSPαL115R/D45A, CSPαL116Δ, and CSPαL116Δ/D45A were lysed on DIV14, subjected to anti-myc immunoprecipitation and immunoblotted to detect co-immunoprecipitation of Hsc70 or ISCU. (d) Lysates from neurons expressing CSPαWT, CSPαWT/D45A, CSPαL115R, CSPαL115R/D45A, CSPαL116Δ, and CSPαL116Δ/D45A were immunoblotted (left) to measure oligomerization and palmitoylation (right) as % of the total protein detected in each lane. Mono = monomer; Dim = dimer; Oligo = oligomers; Palm = palmitoylated. Data shown are from (a) n=6 for CSPα oligomerization, n=9 for CSPα palmitoylation, and n=13 for SNAP-25 levels (n = independent transductions); (b-c) representatives of n=3 immunoprecipitations; (d) n=4 transductions. In (a) and (d) Data represent means ± SEM. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001; n.s. = not significant by two-tailed Student’s t-test. #P<0.05 by Mann-Whitney-Wilcoxon U test. Uncropped blots are shown in Source Data Fig 2.
