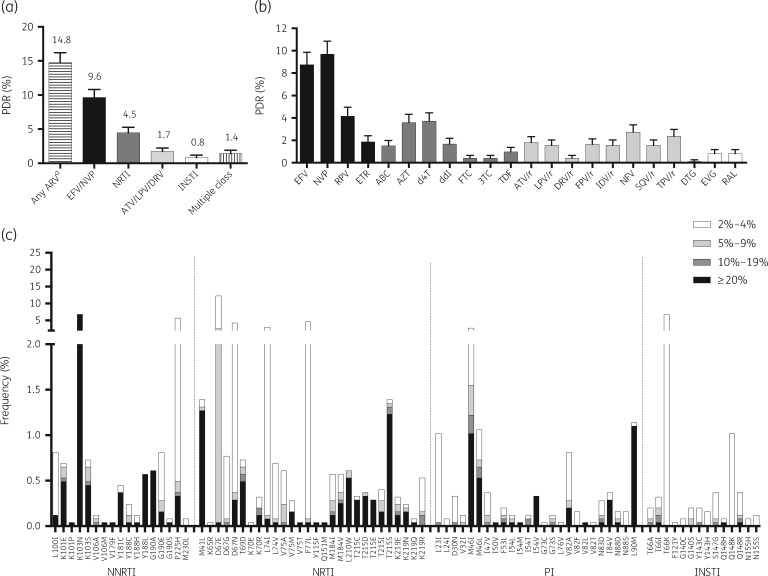
Figure 1.
HIV pretreatment drug resistance (PDR) prevalence in the Mexico City metropolitan area, 2016–18. HIV drug resistance was estimated using next-generation sequencing from 2447 persons starting first-line ART, diagnosed at the Condesa Clinic. (a) HIVDR prevalence by drug class. (b) HIVDR prevalence by antiretroviral drug. Lines represent 95% CI. (c) DRM frequency at different sensitivity thresholds; only surveillance mutations are shown (Stanford HIV Drug Resistance Database). EFV, efavirenz; NVP, nevirapine; RPV, rilpivirine; ETR, etravirine; ABC, abacavir; AZT, zidovudine; d4T, stavudine; ddI, didanosine; FTC, emtricitabine; 3TC, lamivudine; TDF, tenofovir disoproxil fumarate; ATV/r, atazanavir boosted with ritonavir; LPV/r, lopinavir boosted with ritonavir; DRV/r, darunavir boosted with ritonavir; FPV/r, fosamprenavir boosted with ritonavir; IDV/r, indinavir boosted with ritonavir; NFV, nelfinavir; SQV/r, saquinavir boosted with ritonavir; TPV/r, tipranavir boosted with ritonavir; DTG, dolutegravir; EVG, elvitegravir; RAL, raltegravir; INSTI, integrase strand-transfer inhibitor. aConsidering EFV, NVP, any NRTI, ATV/r, LPV/r, DRV/r, DTG, EVG and RAL.