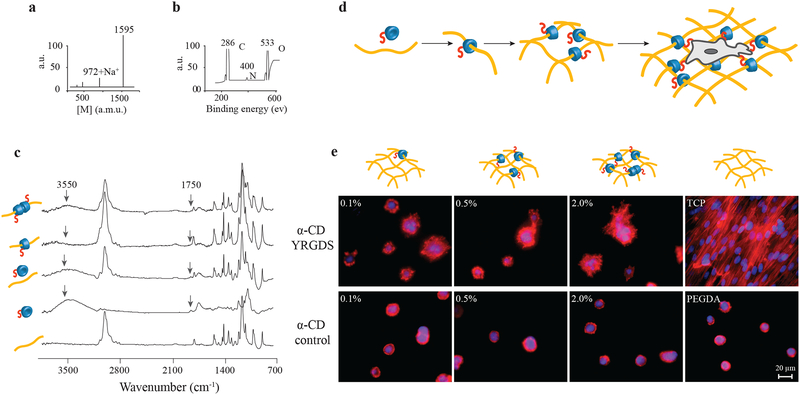
Figure 2. Synthesis and characterization of cell-interactive molecular necklace, α-CD-YRGDS-PEG hydrogels.
a, MALDI-TOF spectrum to confirm peptide conjugation, and synthesis of α-CD-YRGDS (mol wt ~1595 Da) (see in Supplementary Fig. S1a). b, XPS spectrum for α-CD-YRGDS to determine the presence of nitrogen with peptide conjugation to α-CD. c, FTIR spectra for α-CD-YRGDS threading onto PEGDA chains. The spectrum for PEGDA provides a baseline before threading, followed by the α-CD-YRGDS alone, mixed with PEGDA, and α-CD-YRGDS-PEGDA after threading. A peak at 1750 cm−1 arises for the amide stretching of the peptide conjugated to α-CD, and a broader peak at ~3550 cm−1 is for the hydroxyl groups of α-CD and YRGDS. Threading efficiency was ~20% as determined by the ninhydrin assay on rigorously washed and dried hydrogels (see in Supplementary Fig. S1b-d). d, PEGDA (10% w/v) was threaded with α-CD-YRGDS nanobeads and cross-linked to form hydrogels, which were then seeded with MSCs. e, MSCs cultured on the surface of the α-CD-YRGDS-PEGDA hydrogels decorated with varying numbers of α-CD-YRGDS nanobeads (0.1%, 0.5%, and 2.0%, w/v) had significantly greater cell areas and complex actin structures compared to the respective α-CD and PEGDA controls after 4 days of culture (TCP = tissue culture plate; F-actin staining with Texas Red®-X phalloidin).