Figure 4:
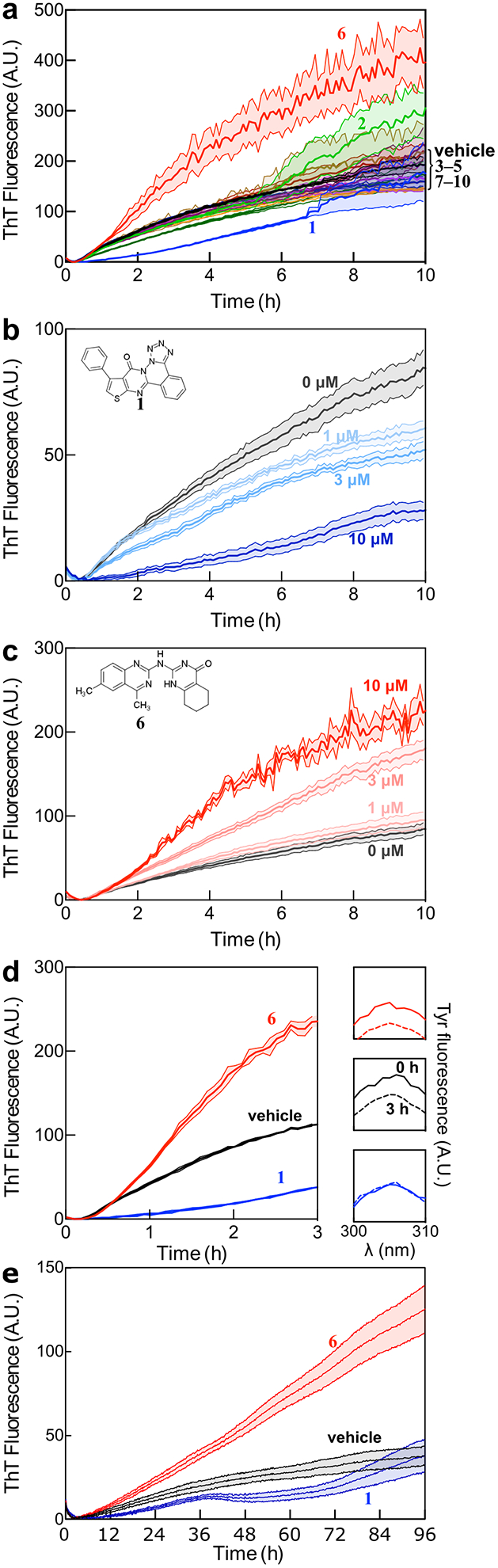
Experimental validation of selected compounds. a) ThT-monitored tau4RD fibril formation kinetics in the presence of 10 compounds selected based on computational screening. Compounds 1 and 6 consistently altered the observed fluorescence kinetics relative to vehicle control. Traces show mean ± SEM of 3 technical replicates, and are representative of three independent experiments. b) Compound 1 extends the lag phase and decreases ThT fluorescence intensity in a dose-dependent manner. c) Compound 6 increases ThT fluorescence in a dose-dependent manner, but does not dramatically affect the lag phase. For B and C, traces show mean ± SEM of 4 technical replicates, and are representative of three independent experiments. d) Measurement of soluble tau content after 3 hours of aggregation indicates that 1 but not 6 affects the extent of aggregation. The main panel shows ThT-monitored aggregation, while the panels on the right show the amount of tau remaining in solution after centrifugation, measured by intrinsic tyrosine fluorescence. Treatment with Compound 1 (blue) shows that soluble tau4RD content is unchanged over the course of the experiment. In contrast, treatments with either Compound 6 (red) or vehicle (black) show a similar loss of fluorescence intensity. e) Compounds 1 and 6 exhibit similar effects on ThT-monitored aggregation of full-length tau, with 1 delaying aggregation and 6 appearing to raise the final level of fluorescence. For D and E, traces show mean ± SEM of 4 or 5 technical replicates respectively, and are representative of at least three independent experiments. Concentration of compounds was set at 10 μM unless otherwise indicated and the concentration of DMSO for all samples was 1%.
