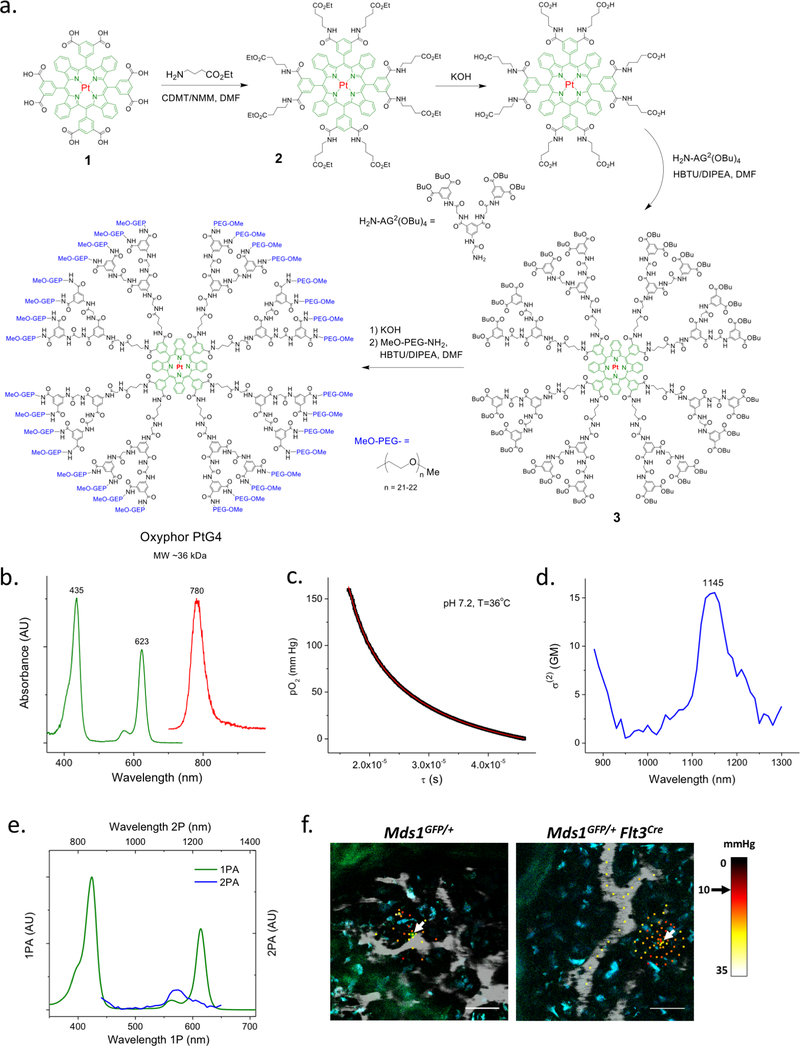
Extended Data Figure 6. Synthesis, structure and characterization of phosphorescent probe Oxyphor PtG4.
The structure of Oxyphor PtG4 is almost identical to that of the previously published probe Oxyphor PdG4 [1], but contains Pt, instead of Pd, at the core of the porphyrin (1: Pt tetra-meso-3,5-dicarboxyphenyl-tetrabenzoporphyrin). a, Synthesis of Oxyphor PtG4. First, eight carboxyl groups on the porphyrin 1 were amended with 4-amino-ethylbutyrate linkers. Upon hydrolysis of the terminal esters in the resulting porphyrin 2, eight aryl-glycine dendrons (H2N-AG2(OBu)4) were coupled to the resulting porphyrin-octacarboxylic acid, giving dendrimer 3. The butyl esters on the latter were hydrolyzed under mild basic conditions, and the resulting free carboxylic acid groups were amidated with mono-methoxypolyethyleneglycol amine (MeO-PEG-NH2, Av MW 1000), giving the target probe Oxyphor PtG4. MALDI-TOF (m/z) was used to confirm the identity of the intermediate products as well as of the target probe molecule. Structure 2 (C116H124N12O24Pt, calculated at MW 2263.85) was found 2264.48 [M]+; structure 3 (C468H540N60O120Pt, calculated at MW 9114.76) was found at 9115.68 [M+H]+ and Oxyphor PtG4 (C1780H3196N92O792Pt, calculated at MW 40538) was found at 35952. For Oxyphor PtG4 we identified an additional peak at MW 66123.6 which is likely due to the presence of dimeric species formed during the ionization process. b, Linear (one photon) absorption (green) and emission spectra (red) of PtG4 in 50 mM phosphate buffer solution (pH 7.2, λex=623 nm. Photophysical constants in PBS, 22oC: e(623)~90,000 M−1cm−1 (molar extinction coefficient), φphos(deox)~0.07 (phosphorescence quantum yield in deoxygenated solution), τair=16μs (phosphorescence decay time on air), tdeox=47ms (phosphorescence decay time in deoxygenated solution). c, Phosphorescence oxygen quenching plot of Oxyphor PtG4. The calibration was performed as previously described [1]. The experimental points were fitted to an arbitrary double-exponential form and thus obtained parametric equation was used to convert the phosphorescence lifetimes obtained in in vivo experiments to pO2 values. d, Two-photon absorption spectrum of PtG4 in deoxygenated dimethylacetamide (DMA, 22oC). e, Arbitrarily scaled one- (green line) and two-photon (blue line) absorption spectra of PtG4. The two-photon absorption (2PA) spectra of PtG4 and of the reference compounds were measured by the relative phosphorescence method, as previously described [2]. The laser source was a Ti:Sapphire oscillator (80 MHz rep. rate) with tunability range of 680–1300 nm (Insight Deep See, Spectra Physics).
All optical spectroscopic experiments and oxygen titrations were performed at least three times, giving highly reproducible results.
f, Representative intravital images of an HSPC (green, left image), MFG-HSC (green, right image), vasculature (gray, Rhodamine-B-dextran 70 kDa), and autofluorescence (blue) overlaid with localized oxygenation measurements. White arrows point at GFP cells. Black arrow points to color representing 10 mmHg. Colored squares represent individual localized oxygen measurement areas. Images represent data from two independent experiments for each mouse model. Scale bars ~50 μm.
[1] Esipova, T. V. et al. Two new “protected” oxyphors for biological oximetry: properties and application in tumor imaging. Anal. Chem. 83, 8756–8765, doi:10.1021/ac2022234 (2011).
[2] Esipova, T. V., Rivera-Jacquez, H. J., Weber, B., Masunov, A. E. & Vinogradov, S. A. Two-photon absorbing phosphorescent metalloporphyrins: effects of p-extension and peripheral substitution. J. Am. Chem. Soc. 138, 15648–15662, doi:10.1021/jacs.6b09157 (2016).