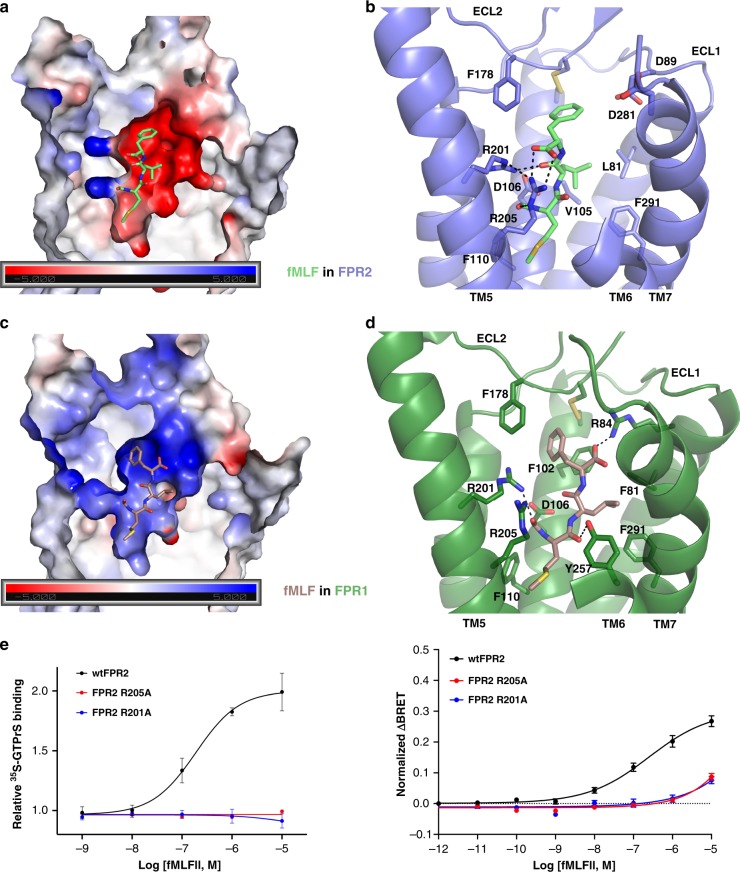
Fig. 4. Peptide-binding pockets in FPR1 and FPR2.
a Negatively charged environment of the ligand-binding pocket in FPR2 and docked fMLF peptide. b Molecular details of the ligand-binding pocket in FPR2 for fMLF. c Positively charged environment of the ligand-binding pocket in FPR1 and docked fMLF peptide. d Molecular details of the ligand-binding pocket in FPR1 for fMLF. Hydrogen-bonding interactions are shown as dashed lines. e Effects of mutations R205A and R201A on fMLFII-induced receptor activation determined by 35S-GTPγS binding assays (left) and BRET-based assays (right). BRET-based assays measured Gi coupling to receptors. n = 3–10, data are mean ± s.e.m. Source data are provided as a Source Data file.