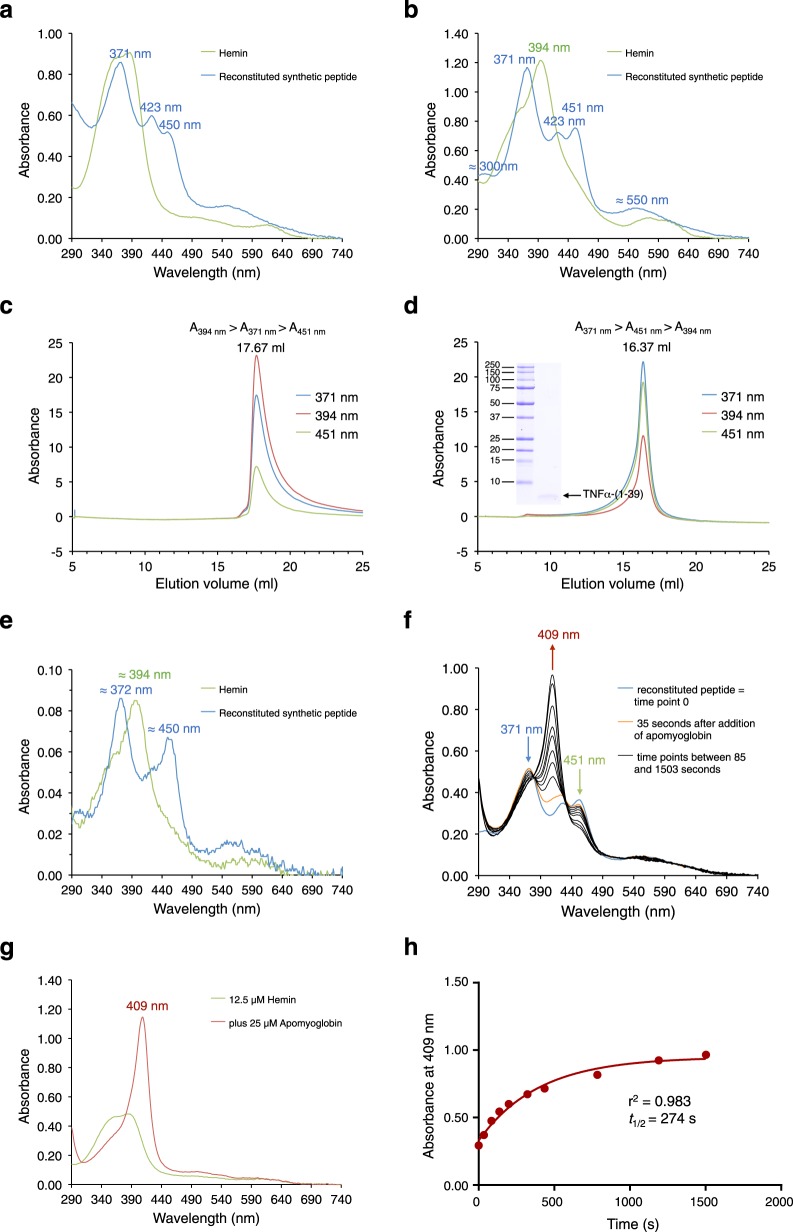
Fig. 4. Hemin reconstitution of synthetic TNFα-(1–39) peptide and heme dissociation by addition of apomyoglobin.
a UV/VIS spectra of 25 µM hemin (green trace) and hemin-reconstituted synthetic TNFα-(1–39) peptide (blue trace) in TN buffer. b UV/VIS spectra of 25 µM hemin (green trace) and hemin-reconstituted synthetic TNFα-(1–39) peptide (blue trace) in TN buffer containing 0.1% Triton X-100. c Gel filtration of hemin (in TN/0.1% Triton X-100) and d of hemin-reconstituted synthetic TNFα-(1–39) peptide (in TN/0.1% Triton X-100) using a Superose 6 Increase column equilibrated in TN buffer containing 0.1% Triton X-100. Elution was followed by absorbance at 371 nm (blue trace), 394 nm (red trace), and 451 nm (green trace). Binding to the synthetic peptide shifts the elution volume of heme from 17.67 to 16.37 ml as confirmed by analysis of the peak fraction by SDS-PAGE (inset in d). e Peak fractions of both gel filtrations were analyzed by UV/Vis spectroscopy (hemin, green trace; hemin-reconstituted peptide, blue trace) confirming stable bis-thiolate ligation of heme by the synthetic peptide. f Hemin-reconstituted synthetic TNFα-(1–39) peptide (containing about 12.5 µM bis-thiolate-ligated heme) (blue trace) was incubated at room temperature with about 25 µM apomyoglobin in TN buffer pH 8.8 and UV/VIS spectra were recorded for the next 25 minutes (orange trace, after 35 s; black traces, time points between 85 and 1503 s). Formation of metmyoglobin led to a decrease of absorbances at 371 (blue arrow) and 451 nm (green arrow) and an increase of absorbance at 409 nm (red arrow). g In a control experiment incubation of 12.5 µM hemin (green trace) with about 25 µM apomyoglobin in TN buffer pH 8.8 immediately led to formation of metmyoglobin with an absorbance of 1.15 at 409 nm (red trace). h The 409 nm (red trace) absorbances of experiment f were plotted against the incubation time. The data were then fitted with the GraphPad Prism program using the exponential one phase decay function giving a decay half-life of t1/2 = 274 s. Three independent reconstitution experiments were performed (a, b and f). f–h show data from one experiment. In a second heme dissociation experiment t1/2 was determined to be 290 s.