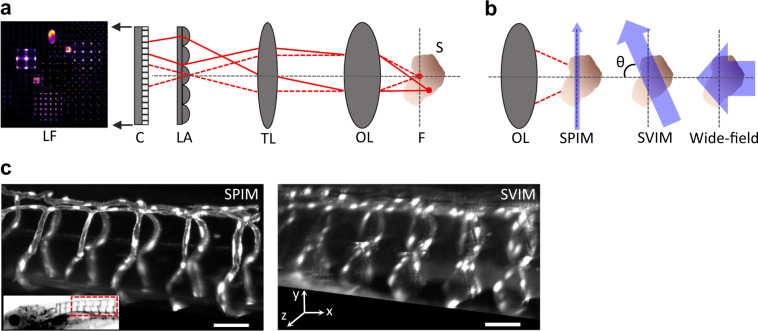
Fig. 1. Selective volume illumination microscopy enhances LFM for the synchronous imaging of 3D samples.
a LFM is a simple extension of a conventional microscope, which produces a magnified image of the sample (S) from the native focal plane (F) to the image plane (IP) using an objective lens (OL,) and tube lens (TL). LFM places a micro-lens array (LA) at the IP, encoding 3D image information into a 2D light-field image (LF), which is captured by a planar detection camera. This permits LFM to synchronously capture information at z-positions above and below F; the 3D image of the sample is reconstructed from the LF image, based on knowledge of the optical transformation. b SVIM improves LFM by selectively illuminating the volume of interest within the sample. This decreases background and increases contrast when compared to wide-field illumination of the entire sample. SVIM was implemented through the use of light-sheet (SPIM) illumination that is scanned axially, so that the thin sheet of excitation is extended into a slab. In our work, the SVIM illumination axis was orthogonal to the detection axis (θ = 900), but the benefits of reduced background can be obtained by using illumination from a different angle, and/or by employing non-linear optical effects to selectively excite the volume of interest. c SPIM and SVIM 3D images of the trunk vasculature of 5 dpf zebrafish larva reveal the compromises between resolution and volumetric imaging time. SPIM offers higher resolution but requires the collection of 100 sequential images to cover the 100-µm-depth z-stack; SVIM captures the same 3D volume in a single snapshot, two-orders-of-magnitude faster, but with lower resolution. Transgenic animal, Tg(kdrl:GFP), had its vasculature fluorescently labeled with green fluorescent protein (GFP). Inset shows the approximate location of the imaged volume along the trunk of the zebrafish larva. Scale bars, 50 µm.