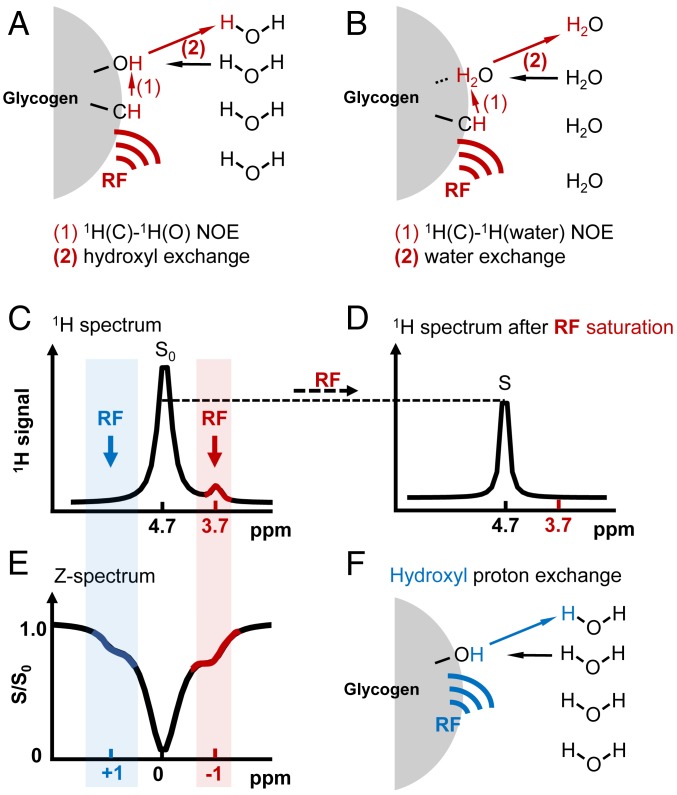
Fig. 1.
Basics of the glycoNOE saturation transfer experiment. (A and B) Two possible NOE-based saturation transfer pathways from glycogen aliphatic protons to water: (A) saturation on glycogen aliphatic protons by a radio frequency (RF) pulse is transferred to a neighboring hydroxyl proton and subsequently to water via proton chemical exchange between glycogen hydroxyl protons and water; (B) saturation on aliphatic protons is transferred directly to a nearby bound water and then to the free water pool via water molecular exchange. (C and D) Schematic 1H MR spectrum for glycogen in H2O before (C) and after (D) the RF saturation of the aliphatic protons. S0 and S represent water intensities in the two spectra. (E) Z-spectrum, showing S/S0 as a function of RF irradiation frequency. Signal shown at around −1 ppm in the Z-spectrum is from glycogen aliphatic protons undergoing the possible NOE-based saturation transfer mechanisms (A and B), while signal at around +1 ppm is from hydroxyl protons undergoing chemical exchange with water (F). Because of multiple saturation transfer events in a single MR sequence, the glycogen signal in the Z-spectrum is enhanced compared to that in the 1H MR spectrum. Note that the Z-spectrum is referenced to the water frequency.