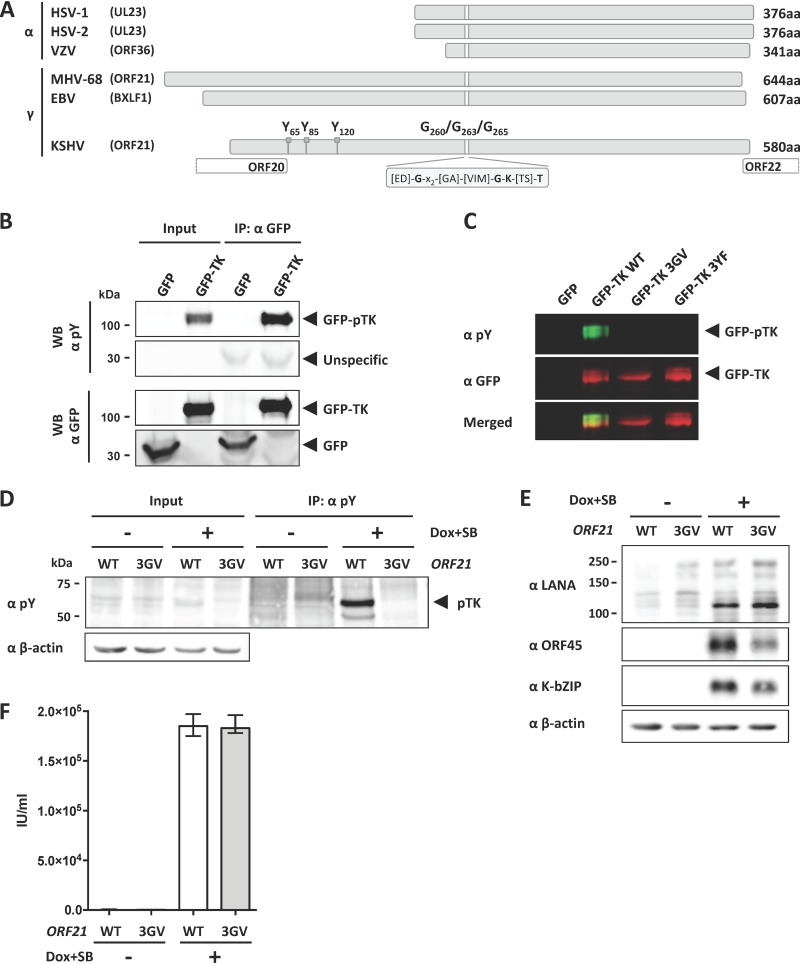
FIG 1.
TK autophosphorylation is not essential for KSHV lytic replication in tissue culture. (A) Graphical representation of the TK genes from alphaherpesviruses HSV-1, HSV-2, and VZV and gammaherpesviruses EBV, KSHV, and MHV-68. Phosphorylated tyrosine residues Y65, Y85, and Y120 and glycine residues G260, G263, and G265 in the ATP binding pocket of KSHV TK are indicated. (B) GFP-TK was overexpressed by transient transfection into HEK293 cells and immunoprecipitated 48 h later using a GFP antibody. Phosphorylation of GFP-TK and total GFP-TK were estimated by immunoblotting (Western blotting [WB]) using a phosphotyrosine antibody and GFP antibody, respectively. (C) GFP or GFP-TK WT, 3GV, or 3YF (see the text) was overexpressed by transient transfection in HEK293 cells. Total lysates were analyzed 48 h after transfection by immunoblotting using antibodies to phosphorylated tyrosine residues or to GFP. (D) iSLK cells stably transfected with either KSHV BAC16 WT or the ORF21/TK ATP-binding pocket mutant KSHV BAC16 3GV (see the text) were induced with Na butyrate (SB) (1 mM) and doxycycline (1 μg/ml) to trigger lytic replication. Nonreactivated cells were used as a control. At 48 h after induction, cells were lysed, immunoprecipitated using beads coupled to an antibody against phosphorylated tyrosine residues, and analyzed by immunoblotting using antibodies against phosphorylated tyrosine (pY) and β-actin. A representative experiment is shown. Similar results were obtained with three independently generated sets of iSLK populations that had been stably transfected with KSHV-WT and KSHV-ORF21_3GV bacmids. (E) At 48 h after induction, iSLK/KSHV-WT and iSLK/KSHV-ORF21_3GV cells were lysed and analyzed by immunoblotting using antibodies against LANA, ORF45, K-bZIP, and β-actin. (F) Supernatants from reactivated and nonreactivated iSLK cells (E) were used to infect HEK293. Infected GFP-positive cells were counted 48 h after infection.