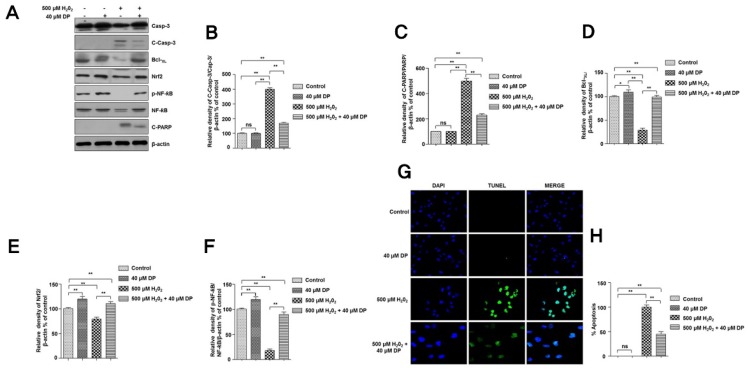
Figure 2.
Delphinidin protects C28/I2 chondrocyte cells from H2O2-induced oxidative stress via nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and nuclear factor kappa B (NF-κB). (A) The C28/I2 chondrocyte cells were treated with 500 µM H2O2 in presence or absence of 40 µM delphinidin (DP) for 4 h. After cell lysis, total cell extracts (30 µg) were separated on 8% or 10% SDS-PAGE and analyzed by Western blotting using primary antibodies against proteins (Bcl-XL, caspase-3, cleaved caspase-3, Nrf2, NF-κB, p-NF-κB, and cleaved poly(ADP-ribose) polymerase N-acetylcysteine (PARP)). β-Actin was used as a loading control. (B–F) The relative amounts of caspase-3/leaved caspase-3, cleaved PARP, Bcl-XL, Nrf2, NF-κB, and p-NF-κB, respectively, shown in Western blot analyses, were quantified by NIH ImageJ software and represented as a graph. Data represent the means (± SD) of three independent experiments (** p < 0.01, * p < 0.05; ns indicates not significant). (G–H) The C28/I2 chondrocyte cells were treated with 500 µM H2O2 in the presence or absence of 40 µM delphinidin (DP) for 4 h. A terminal uridine nick-end labeling (TUNEL) assay was preformed after according to the instructions of the Promega DeadEnd™ Fluorometric TUNEL system kit. The images were captured using a florescence microscope (BX51-DSU; Olympus, Tokyo). Data represent the means (± SD) of three independent experiments (** p < 0.01; ns indicates not significant).