Table 1.
Fatty acids used in the chromatographic method and mass spectral data.
| Compound | Structure | Theoretical Mass [M − H]− |
Measured Mass [M − H]− |
Elemental Composition | MS Rank a | RDB b |
|---|---|---|---|---|---|---|
| 10-HDA |
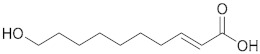
|
185.1183 | 185.1181 MS/MS 139.1122 |
C10H17O3− C9H15O− |
1/1 1/1 |
2 2 |
| 10-Hydroxydecanoic acid |
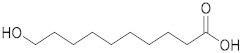
|
187.1340 | 187.1335 MS/MS 141.1286 |
C10H19O3− C9H17O− |
1/1 1/1 |
1 1 |
| 3-Hydroxydecanoic acid |
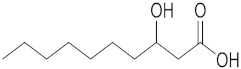
|
187.1340 | 187.1341 MS/MS 59.0155 |
C10H19O3− C2H3O2− |
1/1 1/1 |
1 1 |
| Decanedioic acid |
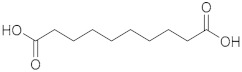
|
201.1132 | 201.1125 MS/MS 139.1133 183.1030 |
C10H17O4− C9H15O− C10H15O3− |
1/1 1/1 1/1 |
2 2 3 |
| 2-Dodecenedioic acid |
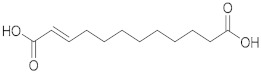
|
227.1289 | 227.1284 MS/MS 183.1398 |
C12H19O4− C11H19O2− |
1/1 1/1 |
3 2 |
| Decanoic acid |
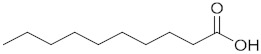
|
171.1391 | 171.1388 MS/MS - |
C10H19O2− - |
1/1 - |
1 - |
| Dodecanoic acid |
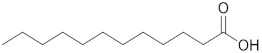
|
199.1704 | 199.1703 MS/MS - |
C12H23O2− - |
1/1 - |
1 - |
a MS Rank: The rank order based on the MS data. This uses a combination of mass accuracy and match to the theoretical isotope pattern. (1st of 1 hit), b RDB: The double bond equivalent that is a formal calculation of the sum of the number of rings and double bonds present in the formula.
