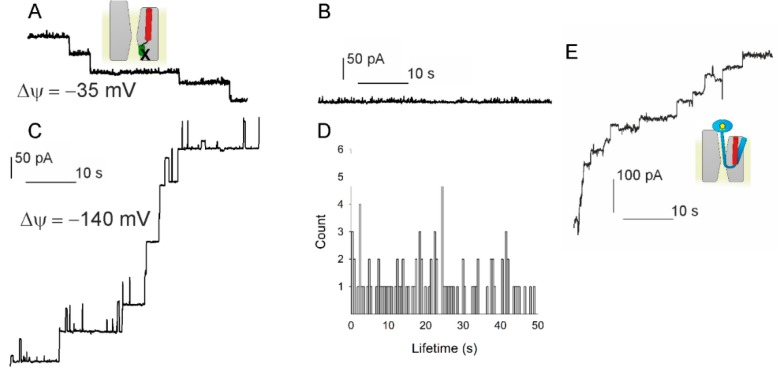
Figure 3.
Testing the hypothesis that voltage driven plug movement confers voltage sensitivity to SecYEG (scheme in the upper left panel). (A) Crosslinking the plug to SecE by 1 mM potassium tetrathionate (KTT) in the SecY(F67C)E(S120C)G mutant forces reconstituted translocons to open. Single channels were recorded at φ = −35 mV. (B) At small φ values the channels virtually do not close. (C) φ = −140 mV elicits a conformational change that closes the channel. Scale bars for A–C are the same. (D) The channel lifetime histogram shows a wide scatter for −140 mV. (E) The plug deletion mutant SecYEG (Δ60–74) retains voltage sensitivity. Channel activity was observed when fusing the mutant containing proteoliposomes in the presence of SecA, ATP, Methotrexate (MTX), and proOmpA-DHFR. MTX binding to DHFR maintains the globular structure of the latter and thus prevents full translocation. φ = −85 mV closes the channels. The schematic representations use the same colour code as in Figure 1: proOmpA in light blue, MTX in complex with DHFR as a yellow pentagon inside a blue ellipse and the cross-link depicted as X. The experiments showed that plug immobilization or removal does not abolish SecYEG’s voltage sensitivity.