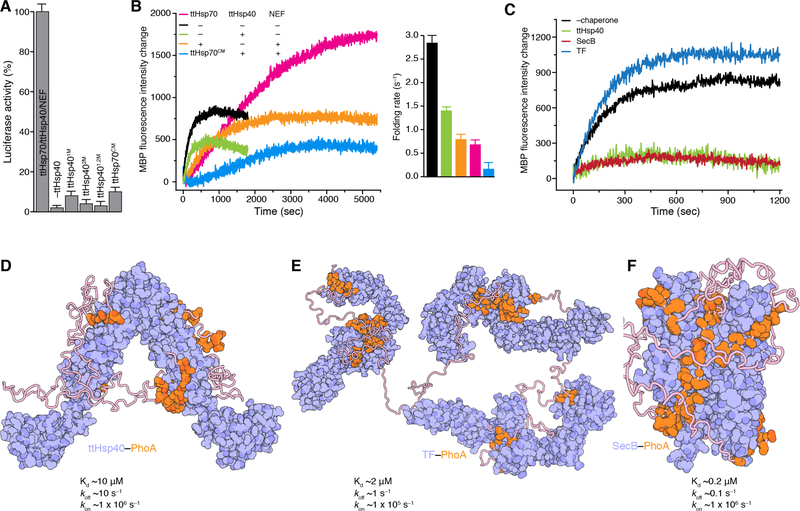
Fig. 4. Chaperone activities and chaperone-client binding architectures.
(A) Folding of denatured luciferase by the ttHsp70/ttHsp40/ttNEF tripartite chaperone machinery measured as percentage of its enzymatic activity. ttHsp40 superscripts 1M, 2M, and 1,2M refer to amino acid substitutions that impair client binding in the CBD1, CBD2 and both CBD1 and CBD2, respectively. Detailed graphs are shown in fig. S15A. Data are mean ± SD. (B) Folding of urea-denatured MBP (pre form) by the tripartite chaperone machinery. The folding rates were determined as described in Materials and Methods. ttHsp70CM refers to the C-tail double substitution (D610-Y611 to A610-A611) that impairs binding to ttHsp40. Folding was monitored by Trp fluorescence. Data are mean ± SD. (C) Folding of urea-denatured MBP (pre form) in the presence of ttHsp40, SecB and TF chaperones. For all experiments the MBP:chaperone ratio is 1:2. (D) Cartoon representation of the structures of ttHsp40–PhoA, (E) TF–PhoA (4), and (F) SecB–PhoA (5). Chaperones are colored in blue and PhoA in orange. The structures display the distinct and very different binding modes that the three chaperones use to engage their client proteins. The Kd and rates of chaperone–PhoA complexes are shown.