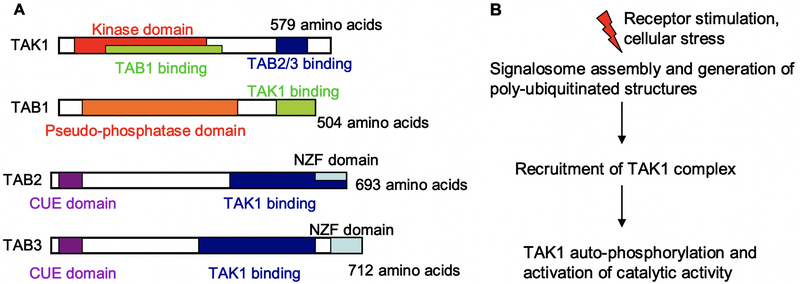
Fig 1. TAK1 architecture and activation.
(A) Schematic representations of the different domains of the constituent molecules of the TAK1 complex, with amino acid length in humans. The kinase activity of TAK1 is regulated by binding interactions with TAB1 and TAB2/3. The latter molecules contain NZF and CUE domains, which bind poly-ubiquitinated (pUb) structures. (B) General outline of TAK1 activation. Receptor stiumuli and cell stress activate signaling pathways that generate pUb structures. The TAK1 complex is recruited to these structures through TAB2/3, resulting in the activation of TAK1 catalytic activity.