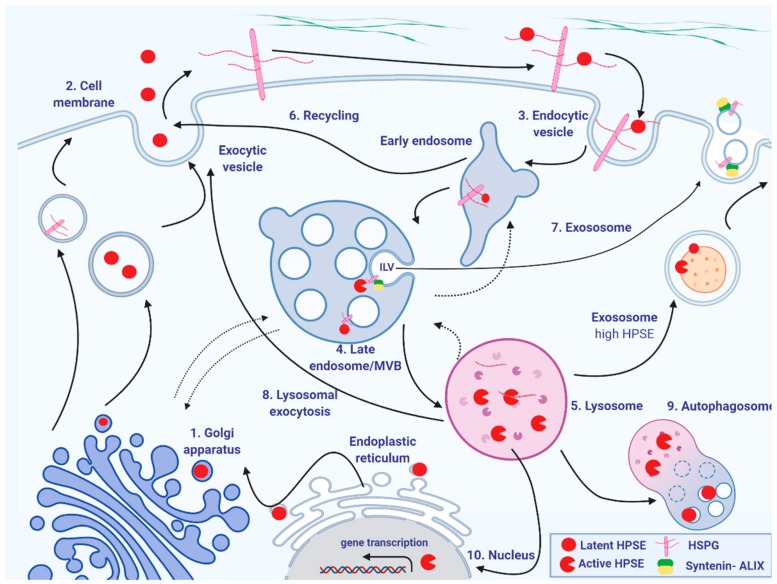
Figure 1.
Schematic model of heparan sulfate proteoglycans and heparanase trafficking. (1) In the Golgi apparatus HS chains are polymerized and preHPSE is processed to produce proHPSE by the elimination of the N-terminal signal peptide. (2) The newly biosynthesized HSPGs are then shifted to the cell membrane where they can interact with the proHPSE and (3) the complex is rapidly internalized by endocytosis and then (4) accumulated in the late endosome. (5) Upon fusion of the late endosome with lysosome, proHPSE is activated and cleaves HS chains that are completely degraded by lysosomal hydrolases. (6) HPSE and HSPGs can be recycled to the cell surface from endosomes. It appears that active HPSE pursues other paths in the cells. (7) Trimming of HS from syndecans by active HPSE present in late endosomes leads to formation of the syndecan-syntenin-ALIX complex [50]. Intraluminal vesicles (ILV) are then formed by the invagination of endosomal membranes, resulting in the formation of multivesicular bodies (MVBs). MVBs release ILVs as exosomes upon fusion with the cell membrane and deliver their cargo to recipient cells. In the presence of high levels of HPSE, the enzyme can be found on the surface of exosomes and modulates tumor microenvironment. (8) Lysosomal exocytosis has been observed in malignant cells. (9) HPSE also regulates autophagy by driving fusion of lysosomes with autophagosomes which degrade macromolecules into monomeric units. (10) Perinuclear lysosomal HPSE can also translocate into the nucleus and regulate gene transcription and cell differentiation.