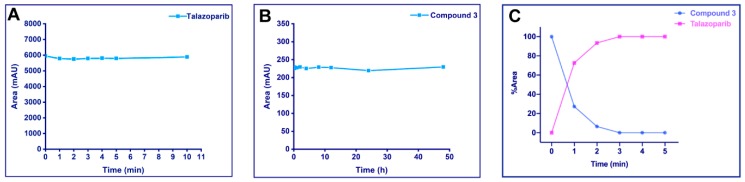
Figure 3.
Stability assays and UV cleavage test in vitro. Compounds were irradiated at 365 nm (4.1 mW/cm2). Samples were determined on a C18 column using a water/acetonitrile (ACN) gradient. The detection wavelength for high-performance liquid chromatography (HPLC) analysis was 220 nm. The peak area or peak area percentage was plotted against the irradiation time. (A) UV stability of Talazoparib: 2.63 mM Talazoparib in methanol were irradiated up to 10 min, and aliquot samples at different time points were analyzed by HPLC. Talazoparib was stable under the described UV irradiation. (B) Phosphate-buffered saline (PBS) stability of the photoactivatable prodrug: 20 μM compound 3 in pH = 7.4 PBS (containing 10% DMSO) were incubated at 37 °C for 48 h and analyzed by HPLC. The peak area of compound 3 did not show significant changes. (C) UV cleavage test on compound 3: 20 μM of the compound in methanol was irradiated at 365 nm for 5 min and analyzed by HPLC. By progressing irradiation, compound 3 was converted into Talazoparib.