Figure 4.
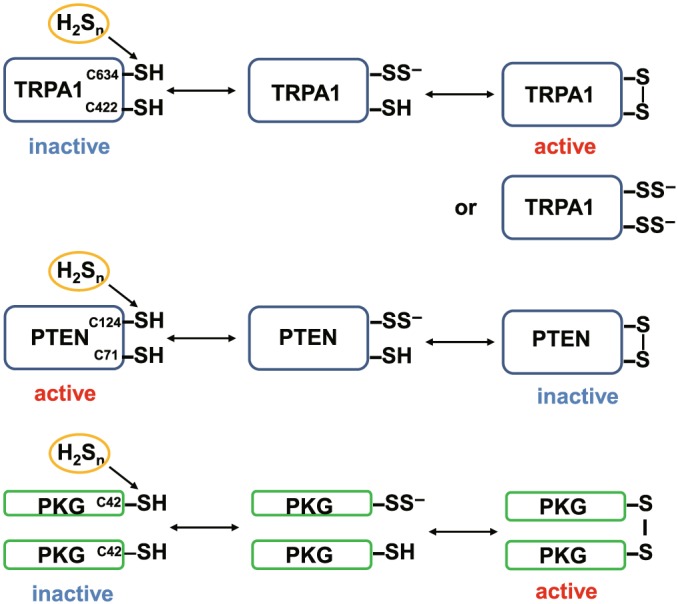
Two cysteine residues are responsible for the regulation of some target proteins by S‐sulfuration. The cys422 and cys634 at the amino terminus of TRPA1 channels are sensitive to H2Sn to induce Ca2+ influx (Hatakeyama et al., 2015; Kimura et al., 2013). Either one of the cysteine residues is S‐sulfurated to react with the remaining thiol to produce a cysteine disulfide bond, which may induce enough conformational changes to modify their activity. Alternatively, both cysteine residues are equally S‐sulfurated. However, compared with the formation of a disulfide bond, it may induce less conformational change to regulate the activity of the channels. When either the cys71 or cys124 of PTEN is S‐sulfurated, a cysteine disulfide bond is produced that then inactivates its activity (Greiner et al., 2013). A dimer formation by each cysteine residue of the monomer protein kinase G1α (PKG1α) activates the enzyme (Stubbert et al., 2014)
