Table 3.
Pharmacokinetic Parameters of Grazoprevir and Elbasvir Following Multiple-Dose Administration of One Tablet of Elbasvir 50 mg/Grazoprevir 100 mg Daily for 10 Days in Healthy Chinese Participants (Per-Protocol Population)
| PK Parameter | Day 1, N = 12 | Day 10, N = 12 | Accumulation Ratio Day 10/Day 1 |
Pseudo Within-Subject %CVa |
|---|---|---|---|---|
| GM (95% CI) | GM (95% CI) | GMR (90% CI) | ||
| GZR | ||||
| AUC0–24, h·µMb | 0.361 (0.26–0.503) | 0.849 (0.605–1.19) | 2.53 (1.79–3.09) | 37.4 |
| Cmax, µMb | 0.0352 (0.0242–0.0513) | 0.0938 (0.0569–0.155) | 2.67 (1.80–3.94) | 53.3 |
| C24, nMb | 6.81 (5.13–9.04) | 13.7 (10.8–17.3) | 2.01 (1.64–2.47) | 27.9 |
| Tmax, hc | 4.00 (2.00–8.00) | 5.00 (2.00–8.00) | – | – |
| t1/2, hd | 30.8 (30.2) | 31.7 (22.8) | – | – |
| EBR | ||||
| AUC0–24, h·µMa | 1.68 (1.48–1.90) | 2.66 (2.12–3.20) | 1.58 (1.37–1.83) | 20.0 |
| Cmax, µMa | 0.137 (0.121–0.156) | 0.202 (0.17–0.24) | 1.47 (1.26–1.72) | 21.4 |
| C24, nMa | 40.4 (34.4–47.4) | 70.1 (57.6–85.2) | 1.74 (1.52–1.98) | 18.0 |
| Tmax, hb | 4.00 (3.00–6.00) | 4.00 (3.00–6.00) | – | – |
| t1/2, hd,e | 17.7 (12.3) | 19.2 (10.8) | – | – |
Notes:aPseudo within-subject %CV = 100 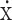 sqrt((Sa2+Sb2-Sab×2)/2), where Sa2 and Sb2 are the estimated variances on the log scale for the 2 days, and Sab is the corresponding estimated covariance, each obtained from the linear mixed-effects model. bBack-transformed least-squares mean and CI from mixed-effects model performed on natural log-transformed values. cMedian (min, max) reported for Tmax. dGM and %CV = 100 × sqrt(exp(s2)-1), where s2 is the observed variance on the natural log scale, reported for t1/2. eOne participant was excluded from statistical calculations (n = 11).
sqrt((Sa2+Sb2-Sab×2)/2), where Sa2 and Sb2 are the estimated variances on the log scale for the 2 days, and Sab is the corresponding estimated covariance, each obtained from the linear mixed-effects model. bBack-transformed least-squares mean and CI from mixed-effects model performed on natural log-transformed values. cMedian (min, max) reported for Tmax. dGM and %CV = 100 × sqrt(exp(s2)-1), where s2 is the observed variance on the natural log scale, reported for t1/2. eOne participant was excluded from statistical calculations (n = 11).
Abbreviations: %CV, percent geometric coefficient of variation; AUC0–24, area under the curve from time 0 to 24 hrs; C24, concentration of the drug at 24 hrs after dosing; Cmax, maximum concentration of the drug; CI, confidence interval; EBR, elbasvir; GM, geometric least-squares mean; GZR, grazoprevir; PK, pharmacokinetics; t1/2, apparent terminal half-life; Tmax, time of maximum concentration.
