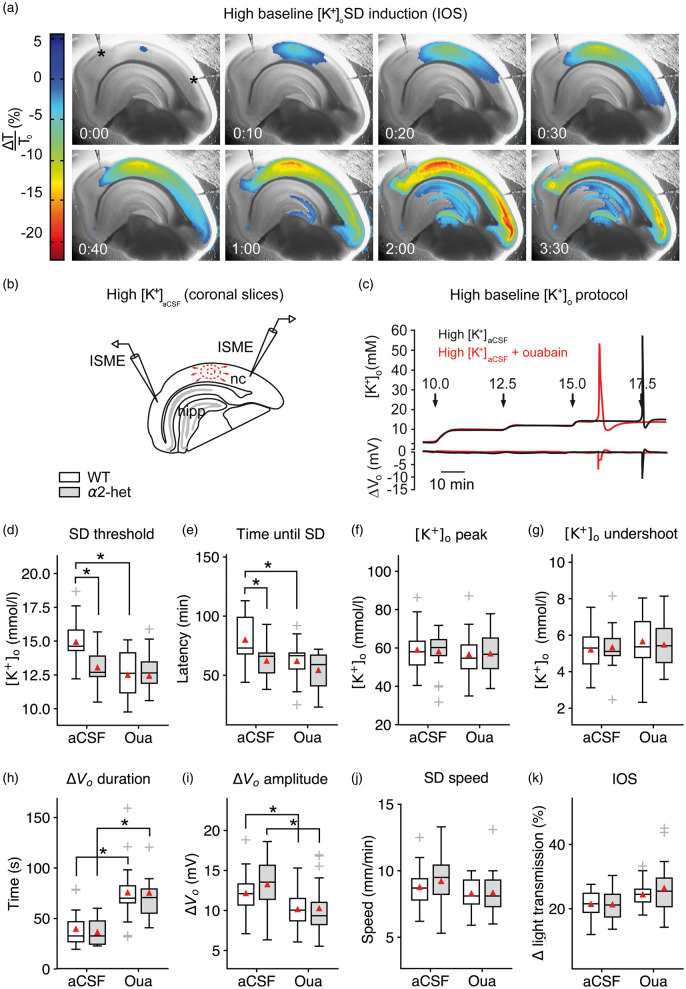
Figure 1.
Na+/K+-ATPase α2 deficiency increases SD susceptibility in acute brain slices. (a–b) SD speed and invaded area were imaged using light transmission changes (IOS). In the interface-type recording chamber, SD is characterized by a short increase (blue), followed by a long-lasting decrease of the IOS (red). Elevated baseline [K+]o induced SD in the neocortex (nc) and frequently in the hippocampus (hipp) but not in the brainstem. (c) Stepwise increased baseline [K+]o allowed for threshold assessment using ISMEs. (d) SD occurred at lower [K+]ACSF in α2+/KOE4 compared to WT. Co-application of 5 µM ouabain did not significantly add to the α2 deficiency effect on threshold [K+]ACSF indicating ouabain action via the astrocytic and not the neuronal isoform. (e) SD latency was shorter in α2+/KOE4 compared to WT mice confirming the lower [K+]o threshold in α2-deficient mice. (f–g) Genetic α2 reduction did not affect [K+]o peak and [K+]o undershoot following SD. (h–i) Only pharmacological inhibition of the α2/3 portion of the Na+/K+-ATPase prolonged SD duration and decreased the DC amplitude irrespective of the genetic background. (j) SD speed was high under elevated [K+]ACSF without additional effects of genetic or pharmacological α2 reduction. (k) IOS amplitude was not affected significantly by genetic or pharmacological α2 reduction. Sample sizes: α2+/KOE4, n = 16–19; α2+/+, n = 21–23. *p < 0.05.