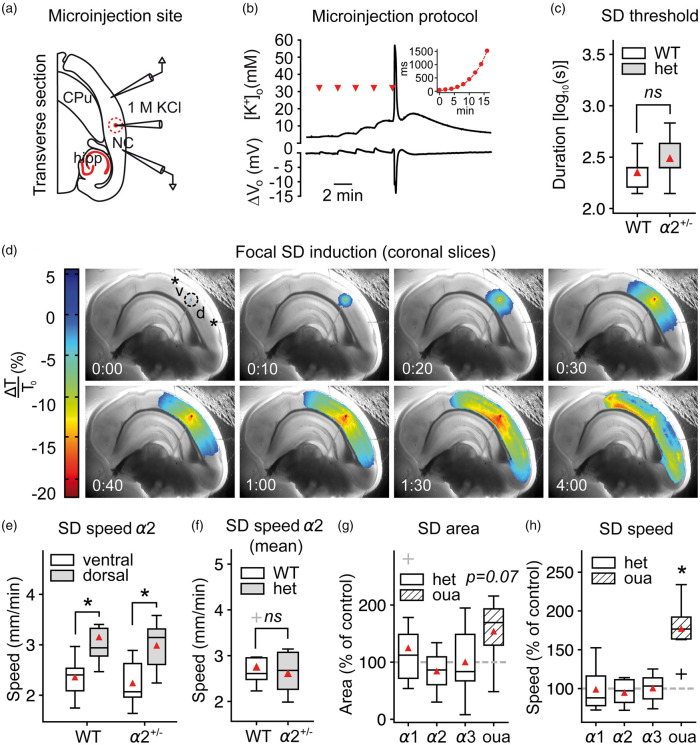
Figure 4.
SD speed is not affected by α2-deficiency in acute brain slices. (a–b) SD was induced focally in acute coronal brain slices under physiological ACSF by microinjecting an increasing volume of 1M KCl into the tissue. NC: neocortex; hipp: hippocampus; CPu: caudate putamen (c) The necessary injected 1M KCl volume to evoke SD was similar in α2+/KOE4 and α2+/+ mice under normal [K+]ACSF (α2+/KOE4: n = 9, α2+/+: n = 6). (d) SD speed and area were calculated from the dorsal (“d”) and ventral (“b”) portion relative to injection point (dashed circle) in coronal brain slices. (e) SD propagated with markedly higher speed in the dorsal compared to the ventral portion of the cortex as measured from the injection site. (f) SD speed was not different between α2+/KOE4 and WT (mean of dorsal and ventral speeds) under normal [K+]ACSF. Data were obtained from 9 α2+/KOE4 (39 SDs, 27 slices) and 9 α2+/+ (37 SDs, 25 slices) mice. (g) The projected area of SD spread on the slice surface tended to be larger (P = 0.07) only in ouabain-treated slices with no effect in α2+/KOE4 mice. (h) Ouabain application increased SD speed drastically (77.3 ± 35.2%, P < 0.00001), whereas genetic α isoform deficiency had no effect on SD speed. Sample sizes for speed and area calculations: α2+/KOE4/α2+/+, n = 9; α3+/KOI4/ α3+/+, n = 9–16; α1+/KOE15/ α1+/+, n = 8; ouabain, n = 7. *P < 0.05.