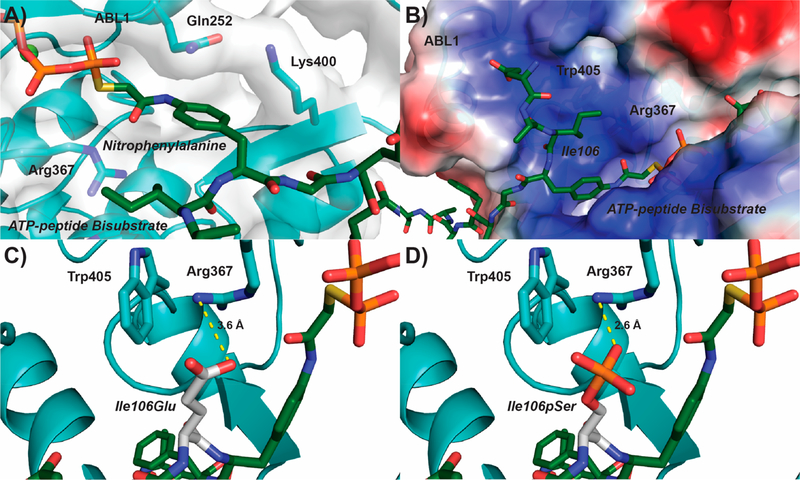
Figure 4.
Structural rationale for negatively charged residues in the seventh CTD position directing ABL1 phosphorylation of Tyr1. (A) Structure of ABL1 (light blue) bound to an ATP-peptide bisubstrate analog (dark green) based on the SRC sequence (PDB: 2G1T). The ABL1 surface is represented as a gray transparent layer around the protein. (B) Electrostatic region of ABL1 preceding the nitrophenylalanine binding pocket (blue−positive, red−negative). (C,D) Modeling of a phosphoserine mimic or phosphoserine (Glu or pSer, respectively; white) into the Ile106 position of the ATP-peptide bisubstrate analog. The most likely rotamer for Glu and pSer was selected for modeling. Predicted salt bridges between the modeled Glu/pSer and Arg367 of ABL1 are shown in yellow.