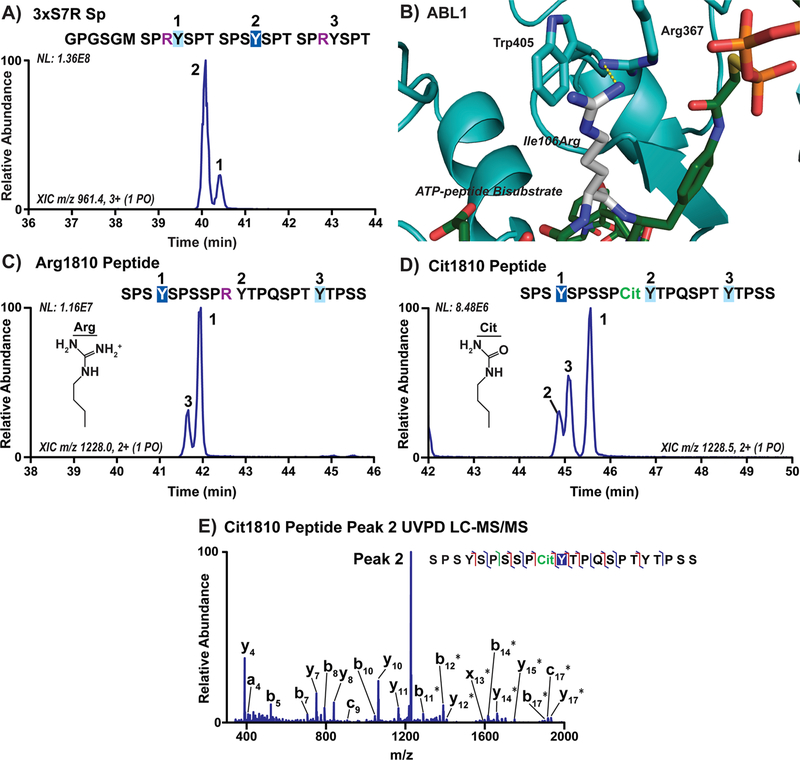
Figure 5.
Arginine and its citrullination in the 7th CTD position alter ABL1 preference for Tyr1 sites. (A) Extracted chromatograms of singly phosphorylated species in three-repeat CTD substrate with Ser7Arg mutations (3xS7R Sp) treated with ABL1. Phosphorylation sites were localized using UVPD-MS and are indicated on the chromatogram by repeat number. The GPGSGM amino acid sequence at the N-terminus is left over from 3C proteolysis of the 6xHis-GST tags prior to mass spectrometry. The sites of major and minor phosphorylation were indicated by dark or light blue boxes, respectively, and the arginine mutations are shown in purple. (B) Structure of ABL1 (light blue) bound to the ATP-peptide bisubstrate analog of SRC (green) with Ile106 modeled as an arginine (white). (C,D) Extracted chromatograms of peptides derived from human distal CTD repeats 30−33 containing either wild-type Arg1810 or citrullinated Arg1810 (Cit1810, green) treated with ABL1. (E) UVPD-MS spectrum of the novel peak in ABL1 treated Cit1810 peptide corresponding to phosphorylation of the middle tyrosine (peak 2).