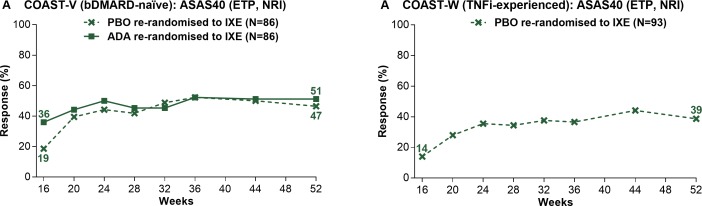
Figure 3.
Proportion of patients initially randomised to PBO or ADA achieving ASAS40 responses on treatment with ixekizumab from week 16 through week 52 in COAST-V (A) and COAST-W (B). ETP population. Missing data were imputed using NRI. ADA, adalimumab; ASAS, Assessment of SpondyloArthritis international Society; bDMARD, biological disease-modifying antirheumatic drug; ETP, dose double-blind extended treatment period; IXE, ixekizumab; NRI, non-responder imputation; PBO, placebo; TNFi, tumour necrosis factor inhibitor.