Abstract
We have introduced a new class of stable organometallic Cr reagents (compounds 1–4) that are readily prepared, yet reactive enough to serve as precursors. They were used for ethylene tetramerization catalysis following stoichiometric activation by in situ protonation. This study highlights the importance of balancing stability with reactivity in generating an organometallic precursor that is useful in catalysis. Moreover, precursor 4 allowed for the isolation and crystallographic characterization of a room-temperature stable cationic species, (PNP)CrR2+ (R = o-C6H4(CH2)2OMe, PNP = iPrN(PPh2)2). This complex (5) may be used as a single component precatalyst, without any alkylaluminum reagents. This result provides an unprecedented level of insight into the kind of structures that must be produced from more complicated activation processes.
Graphical Abstract

INTRODUCTION
Chromium catalysis has not yet experienced a renaissance quite like other first row transition metals.1 Recent development of low-valent Cr catalysis in organic methodology has renewed interest in reactive Cr σ-aryl complexes.2 The necessity for suitable and well-defined organometallic precursors has been appreciated in the context of iron and nickel catalysis.3 Anionic (e.g., aryl, β-diketiminate, or cyclopentadienyl) ligands have proven useful entries for Cr chemistry.4 However, the selection of chromium hydrocarbyl precursors is very limited (vide infra), which may hinder new methodology development.
Chromium has been uniquely demonstrated to serve as a catalyst for ethylene tetramerization, although a completely selective catalyst has remained elusive.5 Significant efforts have identified ligands6 and cocatalysts7 to support ethylene tetramerization, but the pool of Cr precursors has remained small (Figure 1a). These are typically Cr(III) or Cr(II) salts: Cr(acac)3,8 Cr(ethylhexanoate)3,9 CrCl3(THF)3,10 and CrCl2(THF)2 (acac = acetylacetonate, THF = tetrahydrofuran).11 To generate a catalytically active species in situ, these precursors are typically mixed with alkylaluminum cocatalysts such as modified methylaluminoxane (MMAO) in the presence of an auxiliary ligand. The need for harsh activation processes, in terms of high excess and reactivity of Al additives, has impeded rational improvements to catalysis. More problematically, the paramagnetism of relevant Cr intermediates has limited insight into their structure.12
Figure 1.
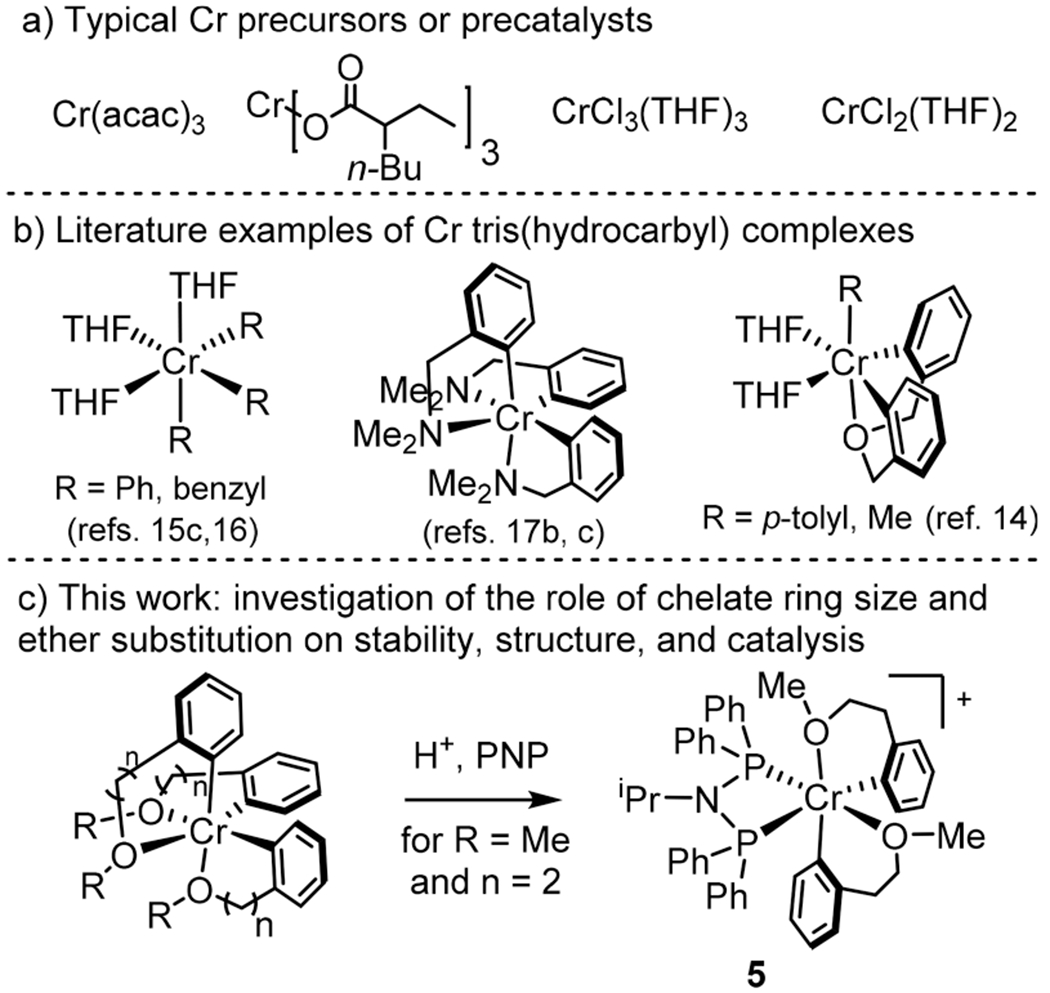
(a) Typical CrIII or CrII precursors or precatalysts, (b) examples of Cr tris(hydrocarbyl) complexes, and (c) this work.
Currently, there are few Cr precatalysts that can be activated by milder methods (or that are self-activating). In several studies for ethylene trimerization13 and in our recent report for ethylene tetramerization,14 catalysis was achieved without excess of alkylaluminum reagents. These and related studies have relied on the isolation of Cr multiaryl or multialkyl complexes.15 The scarcity of examples of “prealkylated” Cr precursors is likely related to the instability of CrPh3(THF)3 and its derivatives.13b,15a,16 The precursor CrBn3(THF)3 (Bn = benzyl) has been reported but is also highly unstable.15c To stabilize Cr-aryl or Cr-alkyl species, chelating ligands have been used in rare cases (see examples in Figure 1b).14,17 However, this strategy has only recently been implemented in chromium catalysis.14,17g
Herein, we report the synthesis of a series of Cr(III) tris(aryl) complexes stabilized by the binding of pendant ethers (Figure 1c). We demonstrate the stability of these complexes, attributable to this chelate effect. Furthermore, these complexes are investigated as precatalysts for ethylene tetramerization following activation with a Brønsted acid in the presence of a diphosphinoamine (PNP) supporting ligand (i.e., stoichiometric activation). Previous studies have suggested that a cationic Cr(III) complex is the product of stoichiometric activation, which is followed by initiation via ethylene insertion, H-transfer, and reductive elimination to generate a Cr(I) active species.13a,b,14 The same type of active species is presumed following activation by alkylaluminum reagents (Scheme 1a), although the involvement of Cr(II) species has not been experimentally ruled out.12 Although precursor Cr(I) cations may be stabilized by carbonyl ligands, such complexes still require activation by alkylaluminum reagents (Scheme 1b).18 Our previous report detailed the first example of activation by protonation (Scheme 1c).14 To our knowledge, no cationic Cr(III) species has yet been isolated and shown to be a viable single-component precatalyst for ethylene tetramerization. Therefore, the identity of the activated Cr species has never been directly established. We demonstrate that by first developing a route to robust but still reactive Cr tris(hydrocarbyl) precursors, an activated complex (PNP)CrR2+ can be isolated and crystallographically characterized (Scheme 1d).
Scheme 1.

(a) Activation of CrCl3-Based Precatalysts with MMAO, Leading to a Reduced Active Species, (b) Activation of Cr(I) Carbonyl Precatalysts with AlR3, Also Leading to a Reduced Active Species, (c) Stoichiometric Activation by Protonation, and (d) Single-Component (PNP)CrR2+ Precatalyst
RESULTS AND DISCUSSION
Synthesis of Cr Tris(aryl) Complexes (1–4) Stabilized by Ether Chelation.
A general procedure for the synthesis of complexes 1–4 was developed (Scheme 2). An amount of 3 equiv of each aryl bromide was converted to the corresponding aryl Grignard reagent by stirring over Mg turnings. The Grignard solutions were used directly in the arylation of CrCl3(THF)3. After filtering away Mg salts, the Cr products could be obtained, typically by precipitation (see Experimental Section for specific workup procedures). In contrast, isolation of the methyl ether-stabilized Cr complex with a single methylene linker in the chelate (R = Me, n = 1) was not successful. The obtained solid residue was completely insoluble in THF or DCM, suggesting that the desired product converted to oligomeric or polymeric forms, possibly due to association of Mg salts. Additionally, although the desired methyl ether-stabilized Cr complex with three methylene linkers in the chelate (R = Me, n = 3) appeared to have been generated in situ, it decomposed in solution at room temperature: the aryl–aryl reductive elimination product was observed by GC/MS in quenched aliquots of this reaction. These changes in reactivity highlight the importance of the chelate ring size and ether substituents in the stabilization of the Cr tris(aryl) complexes.
Scheme 2.

General Synthetic Scheme for Complexes 1–4
Structural Characterization of Complexes 1–4.
Single crystals were obtained of complexes 1, 2, 3, and 4, allowing for structural determination by XRD (Figure 2). Among this series, some structural diversity was observed. For 1 and 4, XRD confirmed the expected geometry of the products as six-coordinate Cr complexes, where each aryl ligand was bidentate due its chelating ether functionality. Complex 2 was determined to be a five-coordinate, square pyramidal complex, where one of the three ether donors was not coordinated to Cr. For 3, a dimeric structure was revealed by XRD, wherein one of the three aryl ligands bridges two Cr centers, binding through the aryl donor to one Cr center, and the ether donor to the other. Under different crystallization conditions (using THF instead of DCM), different crystals were obtained; the XRD analysis revealed a six-coordinate, monomeric Cr center (3′). In 3′, one of the ether donors has dissociated from Cr, and a THF ligand was bound in its place. Clearly, the chelate forming a four-membered ring (in 3) is less favored than the five- and six-membered ring examples (in 1, 2, and 4). An ether donor in 3 prefers either to dissociate in preference to THF or to bridge to another metal center. Nevertheless, the four-membered chelate stabilizes the aryl ligand against reductive elimination relative to the example with the seven-membered ring.
Figure 2.
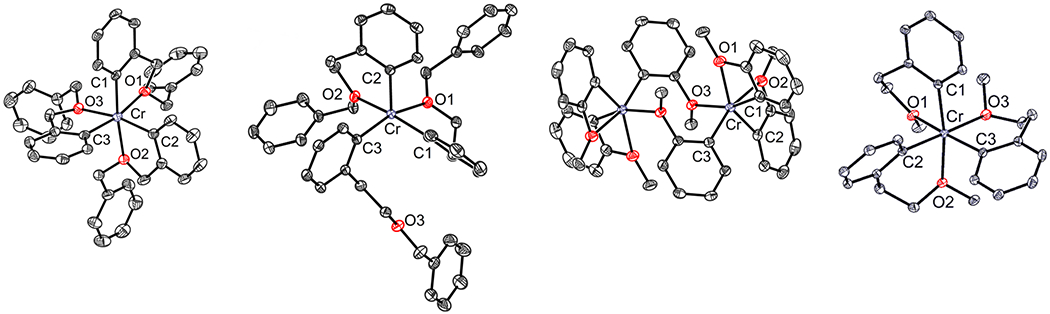
From left to right, solid-state structures of compounds 1, 2, 3, and 4. Thermal ellipsoids are displayed at the 50% probability level. Solvent molecules and hydrogen atoms are omitted for clarity.
Although there were notable differences in the structural arrangement about Cr based on chelate ring size and ether substitution, there are not drastic differences in the bond metrics among the series. However, it can be seen that 4 exhibits the longest Cr–C bonds, by at least 0.03 Å on average (Table 1). This is likely due to it having the largest chelate ring size of the six-coordinate Cr examples. Complex 4 also exhibits relatively long Cr–O bonds, at least 0.02 Å longer than in 1 and 2. However, 3 exhibits the longest Cr–O bonds (excluding the oxygen from the bridging ligand) by 0.06 Å on average. The strained four-membered chelate ring decreases the ability of the ether moiety to bind to Cr. Its propensity to dissociate and/or bind to a different Cr center is further evidence of this. Finally, the O–Cr–C angle (the “bite angle” of the arylether ligands) is close to 90° for 2 and 4, where a six-membered chelate ring is present. Expectedly, a corresponding decrease in the bite angle is seen as the chelate ring size decreases to five (80° in 1) or four (64° in 3).
Table 1.
Selected Bond Lengths and Angles for Compounds 1–5
| bond length (Å) |
bond angle (deg) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| compd | Cr–O1 | Cr–O2 | Cr–O3 | Cr–C1 | Cr–C2 | Cr–C3 | O1–Cr–C1 | O2–Cr–C2 | O3–Cr–C3 |
| 1 | 2.152(2) | 2.176(2) | 2.139(2) | 2.038(2) | 2.040(3) | 2.039(3) | 79.69(9) | 79.81(9) | 79.68(9) |
| 2 | 2.1529(8) | 2.1893(7) | n/aa | 2.050(1) | 2.037(1) | 2.067(1) | 86.41(4) | 90.67(3) | n/aa |
| 3 | 2.230(2) | 2.293(2) | 2.138(1) | 2.032(2) | 2.034(2) | 2.040(2) | 64.81(7) | 63.63(7) | 93.41(7)b |
| 4 | 2.2059(9) | 2.1963(8) | 2.1844(8) | 2.104(1) | 2.081(1) | 2.079(1) | 88.75(4) | 88.05(4) | 88.32(4) |
| 5 | 2.181(1) | 2.108(1) | n/aa | 2.056(2) | 2.072(2) | n/aa | 90.39(6) | 88.02(6) | n/aa |
Not applicable.
O3 in compound 3 is part of the bridging arylether ligand.
Stability of Complexes 1–4.
For use as catalytic or synthetic precursors, Cr multiaryl complexes should exhibit stability in noncoordinating solvents. The commonly used CrPh3(THF)3 is isolable from its synthesis by recrystallization from THF, but its instability in less-coordinating solvents (e.g., diethyl ether or toluene) is well-documented.16a For example, within seconds of adding diethyl ether or toluene to CrPh3(THF)3, conversion to brown precipitate is observed, in the absence of excess amounts of a coordinating ligand such as THF. The decomposition pathway involves reductive elimination to generate biphenyl.16a For comparison, the stability of complexes 1–4 was tested in dried, degassed toluene solution (4 mM) in sealed cuvettes. Over 24 h at room temperature, no changes to the UV/vis absorption spectra were observed for complexes 1, 2, and 4; quenched aliquots analyzed by GC/MS showed no decomposition by aryl–aryl reductive elimination over this time. Compound 3 decomposed slowly over 24 h in toluene at room temperature (a 23% decrease in absorption at 534 nm); dark precipitate was observed from the red solution (λ: 444 nm, 534 nm); reductive elimination was observed by GC/MS. Unsurprisingly, these compounds were not stable in solution upon exposure to air. Clearly, the presence of ether chelation in ring sizes of five or six stabilized the Cr-aryl motif dramatically. With a chelate ring size of four (in complex 3) or with a combination of chelated and nonchelated aryl ligands (as noted in our previous report),14 a lesser degree of stabilization is imposed, although these examples are still more robust than CrPh3(THF)3.
Catalytic Utility of Cr Tris(aryl) Complexes 1–4.
The utility of the Cr complexes reported herein as precursors in catalysis was investigated in the context of selective ethylene tetramerization. Due to structural similarities to previously reported Cr multiaryl complexes, we expected these to be successfully activated by protonation with HBAr′4 in the presence of a PNP ligand.13a,b,14 Using this process, all complexes investigated led to some productivity in the absence of alkylaluminum activators; however, 4 was particularly active (see Table 2). We propose that the differences in productivity are related to the initiation rates (leading to a reduced Cr active species). All of these Cr tris(hydrocarbyl) precursors are expected to generate, upon protonation in the presence of PNP ligands, cationic (PNP)Cr-bis(aryl) species capable of catalysis.
Table 2.
Comparison of Ethylene Oligomerization Catalysis Using Cr Complexes 1–6a
| entry | Cr sourceb | productivity (g/g of Cr) | PEc,d | C6d | 1-C8d | C10–C14d | % 1-C6 in C6d | 1-octene/1-hexenee |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 12 | 0 | 42 | 58 | 0 | 94 | 1.1 |
| 2 | 2 | 62 | 0 | 42 | 58 | 0 | 91 | 1.1 |
| 3 | 3 | 34 | 0 | 72 | 26 | 2 | 98 | 0.28 |
| 4 | 4 | 1500 | 0 | 48 | 45 | 7 | 92 | 0.77 |
| 5 | 5 | 3400 | <1 | 47 | 38 | 15 | 93 | 0.64 |
| 6 | 6 | 0 | 0 | |||||
| 7f | CrCl3(THF)3 | 5200 | <i | 43 | 41 | 16 | 89 | 0.80 |
No alkylaluminum activators were used in these trials (entries 1–6). A comparison is made with MMAO-activated CrCl3(THF)3 (entry 7). Reaction vessel: glass Fisher-Porter bottle. [Cr] = 1 mM. Solvent: 7.5 mL of PhCl. Pressure: 100 psig C2H4. Temperature: 25 °C. Reaction time: 45 min.
Complexes 1–4 and 6 were activated with 1.0 equiv of HBAr′4 in the presence of 1.1 equiv of iPrPNP.
PE = polyethylene.
Wt % (total).
Molar ratio.
Result from ref 14: 300 equiv of MMAO were added in the presence of 1.1 equiv of iPrPNP; other conditions are the same.
The difference in performance is likely related to how the initiation rate is affected by the chelate ring size and substituent on the ether donor. Complex 4 has relatively long Cr–C and Cr–O bonds compared to the other precursors (vide supra), a possible explanation for faster initiation. Importantly, we found that a known Cr tris(aryl) precursor (6, Cr(o-(Et2NCH2)-C6H4)3)19 stabilized by pendant amines was not a viable precatalyst. No oligomers were observed following stoichiometric activation of 6. Likely, the amine donor chelates too strongly to Cr, preventing catalyst formation in terms of efficient protonation, coordination by PNP, or subsequent initiation steps. This difference in behavior highlights the necessary balance between stability and reactivity in these Cr precursors. While stability is desirable in a versatile precursor, sufficient reactivity is still necessary for catalytic utility. The ether chelates employed here satisfy both requirements.
Synthesis and Structural Characterization of 5.
The ability of ligands relevant to catalysis to displace the chelating donors in 1–4 was investigated, but no evidence of a reaction was observed at room temperature, even with bis(diphenylphosphino)benzene (tracked by EPR or UV/vis spectroscopy). Nevertheless, PNP must bind to Cr after the protonolysis of an aryl group in order to generate active catalysts as demonstrated in Table 2. Therefore, the isolation of the cationic complex was targeted. Addition of [H(OEt2)2][BAr′4] (Ar′ = 3,5-(CF3)2-C6H3) to a mixture of iPrN(PPh2)2 (iPrPNP) and 4 results in a color change from orange to green. The product was isolated as a green powder and was proposed to have the formulation of [(iPrPNP)Cr(o-(CH3O(CH2)2)-C6H4)2][BAr′4] (5, Figure 3). This corresponds to protonation of one aryl ligand (releasing 2-methoxyethylbenzene) and binding of iPrPNP to Cr. This solid could be used directly in catalysis simply by dissolving it in chlorobenzene and adding ethylene (vide infra). This product (5) was not readily amenable to crystallization due to its propensity to form oils. However, suitable single crystals of 5 were obtained from DCM. The expected structure was confirmed by XRD: six-coordinate, cationic Cr with two arylether ligands and one PNP ligand bound (Figure 3).
Figure 3.
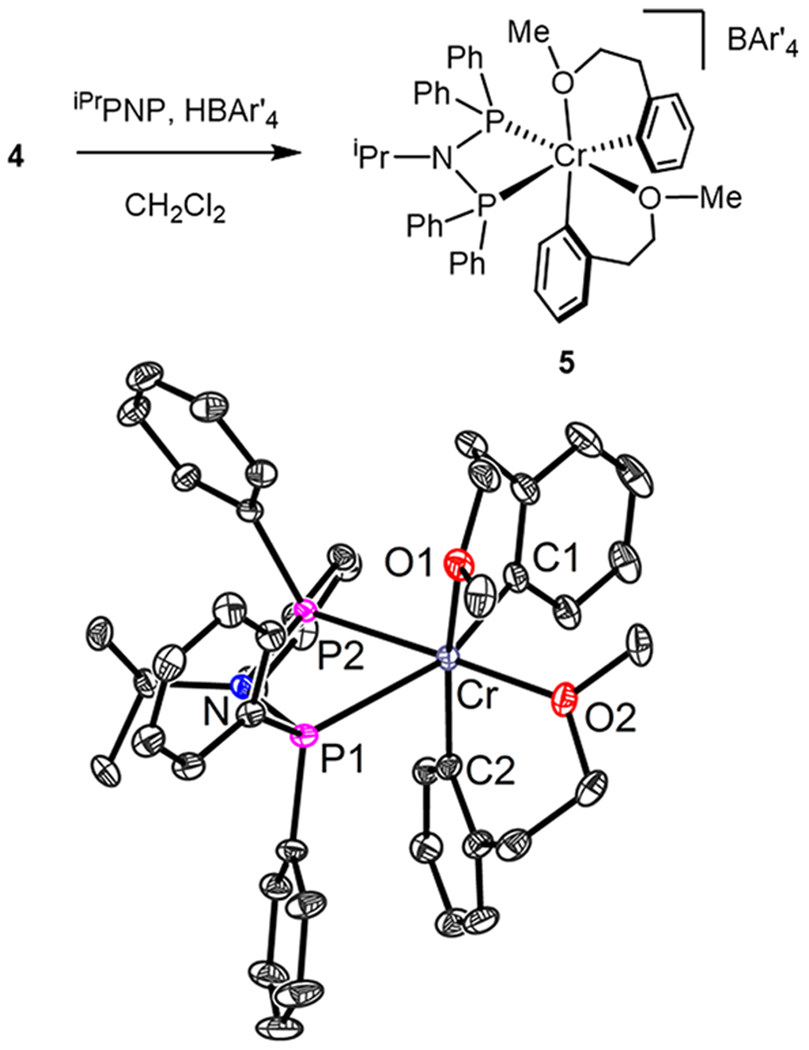
Top: Synthesis of complex 5. Bottom: Solid-state structure of complex 5. Thermal ellipsoids are displayed at the 50% probability level. Solvent of crystallization (CH2Cl2), BAr′4 anion, and hydrogen atoms are omitted for clarity.
The stability of compound 5 in toluene was checked to evaluate that it is practically useful. No changes to the UV/vis absorption spectra were observed in toluene solution for at least 24 h at room temperature, which is very notable given the scarcity of Cr-hydrocarbyl cationic species.15b,20
Indeed, compound 5 is a particularly uncommon example of an isolated Cr-hydrocarbyl cation in the context of ethylene oligomerization catalysis. Although related cationic complexes have in some cases been structurally characterized,12b,18,21 to our knowledge they are not catalytically active without alkylaluminum-based cocatalysts. None of the referenced examples maintain salient features present in 5, which is free of halide or carbonyl ligands, has a single PNP ligand, and has a noncoordinating anion. Because of these features, compound 5 is poised to generate the catalytically active species simply upon addition of ethylene.
Use of Pre-”Activated” Cr Complex 5 in Catalysis.
We found that 5 was a single-component precatalyst for ethylene tetramerization. Following dissolution of 5 in chlorobenzene, addition of ethylene in a high-pressure reaction vessel led to formation of 1-hexene and 1-octene, similar to catalytic trials following stoichiometric activation of 4 (see Table 2, entry 5). Remarkably, the catalytic productivity and 1-octene selectivity are comparable to when CrCl3(THF)3 is activated with 300 equiv of MMAO (Table 2, entry 7). The direct utility of 5 is advantageous since no weighing or premixing of multiple components is necessary prior to loading the reactor. In a traditional activation scheme, the PNP ligand, Cr precursor, and MMAO solution must all be meticulously prepared and combined. It has been reported that MMAO-activation leads to Cr species that are unstable and in which multiple species are detectable by UV/vis and EPR spectroscopy.12c Because 5 is reasonably stable at room temperature, these complications regarding catalyst preparation and activation have been completely removed. Fundamentally this catalytic result also bolsters the assertion that a cationic (PNP)Cr dialkyl complex is the relevant product of traditional MMAO-activation leading to the Cr active species.
CONCLUSIONS
A high degree of stability is imparted to the Cr tris(aryl) motif by the addition of pendant ether donors. However, these precursors (1–4) remain reactive enough for catalytic use in ethylene tetramerization. Differences in stability and reactivity were observed among the series of ether-stabilized Cr precursors. In particular, one example (4) not only led to higher productivity in ethylene tetramerization catalysis but also was a useful synthon for a cationic Cr complex (5). This example (5) is the first single-component precatalyst for ethylene tetramerization and is a rare example of a structurally characterized Cr σ-aryl cationic species. Complex 5 exemplifies structural features required for an “activated” Cr species, eliminating speculation as to the role of MMAO as an activator in typical catalytic processes. Compound 5 represents a unique example of a well-defined and structurally characterized (PNP)CrR2+ activated species, typically produced from MMAO-activation of CrX3-based (X = Cl or acac) precatalysts. Analogous methodologies are expected to be fruitful for other Cr catalytic systems, using the precursors reported here.
EXPERIMENTAL SECTION
Synthesis of Cr Compounds.
See Supporting Information for general notes about methods and materials used.
Cr(o-(C6H5CH2OCH2)-C6H4)3 (1).
A solution of 1-((benzyloxy)-methyl)-2-bromobenzene (0.930 g, 3.36 mmol) in 20 mL of THF was stirred over excess activated Mg turnings at room temperature. After several hours, Grignard formation was complete. The solution was filtered through glass wool, away from excess Mg. It was added dropwise over 10 min to a thawing 20 mL THF suspension of CrCl3(THF)3 (0.420 g, 1.12 mmol). The resulting brown, homogeneous solution was warmed to rt over 90 min, then diluted to 80 mL with Et2O. Next, 0.8 mL of 1,4-dioxane was added; the solution continued to stir at rt for 20 h. The resulting brown solution was filtered through Celite, away from pale, yellow solids. These solids were rinsed into a separate flask using 20 mL of DCM to obtain a yellow-orange solution from insoluble, gray solids. This filtrate was reduced in vacuo to a yellow powder (0.265 g, 0.411 mmol, 37% yield). Yellow single crystals suitable for XRD were grown by cooling a diethyl ether solution of 1 to −40 °C for several days. μeff = 4.0(2) μB (average of three measurements). 1H NMR (400 MHz, CD2Cl2) δ (ppm): 26.6 (br), 11.7 (br), 7.6 (br), 7.3 (br). UV–vis [THF; λ, nm (ε, M−1 cm−1)]: 244 (3.9 × 104), 314 (1.1 × 103), 388 (3.6 × 102), 458 (3.4 × 102). Anal. Calcd for C42H39CrO3: C, 78.36; H, 6.11; N, 0.0. Found: C, 78.18; H, 6.14; N, 0.0.
Cr(o-(C6H5CH2O(CH2)2)-C6H4)3 (2).
A solution of 1-((benzyloxy)-ethyl)-2-bromobenzene (1.81 g, 6.22 mmol) in 10 mL of THF was stirred over excess activated Mg turnings at room temperature. After several hours, Grignard formation was complete. The solution was filtered through glass wool, away from excess Mg, and diluted to 25 mL with THF. The solution was added dropwise over 10 min to a thawing 10 mL THF suspension of CrCl3(THF)3 (0.778 g, 2.08 mmol). The resulting green, homogeneous solution was warmed to rt over 2 h, then diluted to 70 mL with Et2O. Next, 1.2 mL of 1,4-dioxane was added; the solution continued to stir at rt for 24 h. The resulting green solution was filtered through Celite, away from white solids. This filtrate was reduced in vacuo to a green sticky residue, which was redissolved in 5 mL of toluene. This toluene solution was stirred vigorously, and 25 mL of pentane was added to precipitate reddish powder among a sticky, dark green residue. The red powder was collected, and the green residue was redissolved in toluene, and pentane was added in like fashion to precipitate more red powder. These two fractions were combined to give 750 mg of red power, redissolved in 20 mL of toluene to give a green solution, and rinsed from white solids (presumably magnesium salts). This green solution was reduced in vacuo to a green powder (0.613 g, 0.895 mmol, 43% yield). Green single crystals were grown by slow concentration of a toluene solution of 2 under vacuum. μeff = 3.9 μB. 1H NMR (400 MHz, CD2Cl2) δ (ppm): 25.1 (br), 14.9 (br), 7.5 (br), 7.3 (br), 2.4 (s), −17.0 (br). UV–vis [THF; λ, nm (ε, M−1 cm−1)]: 259 (1.9 × 104), 359 (6.1 × 102), 404 (2.9 × 102), 481 (1.5 × 102). Anal. Calcd for C45H45CrO3: C, 78.81; H, 6.61; N, 0.0. Found: C, 79.03; H, 6.70; N, 0.0.
Cr(o-(CH3O)-C6H4)3 (3).
A solution of 2-bromoanisole (1.863 g, 9.96 mmol) in 20 mL of THF was stirred over excess activated Mg turnings at room temperature. After several hours, Grignard formation was complete. The solution was filtered through glass wool, away from excess Mg. It was added dropwise over 5 min to a thawing 30 mL THF suspension of CrCl3(THF)3 (1.244 g, 3.32 mmol). A homogeneous, dark red solution resulted after warming to rt over 3 h. The solution was diluted to 100 mL with Et2O, and 3 mL of 1,4-dioxane was added, causing formation of some precipitate. After stirring at rt for 14 h, a red solution was filtered from light solids using Celite. The filtrate was reduced in vacuo to red, flaky solids, which were redissolved in 10 mL of DCM. The red solution was filtered through glass wool from minimal gray solids, layered with 10 mL of hexanes, and stored for 6 days at −40 °C. Then, the cold supernatant was decanted from ~50 mg of red solids and reduced in vacuo to 12 mL, causing additional precipitation. This suspension was stored at −40 °C for another day. The supernatant was then decanted from red solids, which were dried in vacuo (0.748 g). Satisfactory elemental analysis could not be obtained, possibly due to remaining magnesium salts. Single crystals suitable for XRD could be obtained by vapor diffusion of pentane into a DCM solution at −40 °C.
Cr(o-(CH3O(CH2)2)-C6H4)3 (4).
A solution of 1-bromo-2-(2-methoxyethyl)benzene (1.356 g, 6.31 mmol) in 20 mL of THF was stirred over excess activated Mg turnings at room temperature. After several hours, Grignard formation was complete. The solution was filtered through glass wool, away from excess Mg. It was added dropwise over 15 min to a thawing 30 mL THF suspension of CrCl3(THF)3 (0.791 g, 2.11 mmol). The resulting dark red, homogeneous solution was warmed to rt over 3 h, then diluted to 100 mL with Et2O. Next, 1.2 mL of 1,4-dioxane was added, resulting in a suspension of red solids, which was stirred at rt for 20 h. The resulting suspension was filtered, collecting red solids on a Celite filter cake. These solids were rinsed into a separate flask using 40 mL of DCM. This red DCM solution was reduced in vacuo to yield the product as a red powder (0.192 g, 0.419 mmol, 20% yield). Single crystals suitable for XRD were grown by vapor diffusion of pentane into a THF solution of 4 at −40 °C. μeff = 3.8 μB. 1H NMR (400 MHz, CD2Cl2) δ (ppm): 23.2 (br), 17.4 (br), −14.9 (br). UV–vis [THF; λ, nm (ε, M−1 cm−1)]: 256 (2.7 × 104), 371 (9.9 × 102), 416 (5.0 × 102), 602 (1.5 × 102). Anal. Calcd for C27H33CrO3: C, 70.88; H, 7.27; N, 0.0. Found: C, 70.63; H, 7.37; N, 0.19.
[(iPrPNP)Cr(o-(CH3O(CH2)2)-C6H4)2][BAr′4] (5).
A solution of 4 (0.145 g, 0.317 mmol) and iPrPNP (0.135 g, 0.316 mmol) was prepared in 4 mL of DCM. To the room temperature orange solution, a 4 mL DCM solution of HBAr′4 (0.320 g, 0.316 mmol) was added dropwise over 5 min. Upon completion of addition, a dark green solution was obtained. After 20 min, the solution was reduced to a sticky green solid under vacuum; the dry residue was further dried under vacuum for several hours. The residue was dissolved in minimal DCM (≈1 mL). Hexanes was added in portions to the thick, vigorously stirring solution, causing some oiling, then eventual precipitation of dry green solids. These green solids were isolated by decanting the supernatant and reducing further under vacuum to complete dryness (0.480 g, 0.298 mmol, 94% yield). Single crystals suitable for XRD were grown in ≈1 day by slow evaporation of a DCM solution into hexamethyldisiloxane (HMDSO) at room temperature. μeff = 3.6 μB. 1H NMR (400 MHz, CD2Cl2) δ (ppm): 28.4 (br), 25.6 (br), 12.7 (br), 9.7 (br), 7.8 (s, aryl-H on BAr′4), 7.6 (s, aryl-H on BAr′4), 6.9 (br), 2.9 (br), 0.2 (br), −0.6 (br), −9.3 (br), −17.6 (br). 19F NMR (376 MHz, CD2Cl2) δ (ppm): −62.7 (s). 31P NMR (162 MHz, CD2Cl2) δ (ppm): silent. UV–vis [DCM; λ, nm (ε, M−1 cm−1)]: 619 (2.3 × 102). Anal. Calcd for C77H61BCrF24NO2P2: C, 57.34; H, 3.81; N, 0.87. Found: C, 57.36; H, 3.99; N, 0.92.
Cr(o-(Et2NCH2)-C6H4)3 (6).
This has been described previously,19 and is related to the dimethylamino-substituted version.17b,c Our synthesis is as follows: N-(2-bromobenzyl)-N-ethylethanamine (0.288 g, 1.20 mmol) in 4 mL of THF was stirred over excess activated Mg turnings at room temperature. After several hours, Grignard formation was complete. The solution was filtered through glass wool, away from excess Mg. It was added dropwise over a few minutes to a thawing 4 mL THF suspension of CrCl3(THF)3 (0.149 g, 0.398 mmol). The resulting dark solution was warmed to rt over 2 h, then diluted to 16 mL with Et2O. Next, 0.5 mL of 1,4-dioxane was added, resulting in the precipitation of white solids. After stirring for 18 h, the dark solution was filtered through Celite, and the filtrate was reduced in vacuo to obtain red crystals among a sticky brown residue. This mixture was rinsed with minimal hexanes, then Et2O, decanting the brown washes from the red crystals. Anal. Calcd for C34H51CrN3: C, 73.74; H, 9.28; N, 7.59. Found: C, 73.52; H, 9.18; N, 7.70.
Oligomerization Catalysis.
Complexes 1–4 and 6 were activated as follows: the Cr complex (8.0 μmol) and iPrPNP (8.8 μmol) were dissolved in 1.0 mL of PhCl in a 20 mL vial in the glovebox. To the stirring, room temperature solution, HBAr′4 (8.0 μmol) dissolved in 0.5 mL of PhCl was added dropwise over 1 min. For most examples, a color change rapidly occurred. Quickly, the solution was diluted to 7.5 mL by addition of PhCl and then transferred to a glass Fisher-Porter bottle equipped with a stir bar (for experiments at 100 psi). The reactor was sealed and taken out of the glovebox to the high-pressure setup and placed in a water bath at 25 °C. The gas line was evacuated, then backfilled with ethylene gas. The line was pressurized to 100 psig with ethylene and then opened to the reactor. The pressurized solution was stirred for 45 min. After this time, the reactor was vented, and 0.1 mL of methanol was added to quench the mixture. Adamantane was added to the solution as a reference compound, which was then filtered and analyzed by GC/FID to quantify the oligomers. Polymer was weighed on a tared glass fritted filter.
For catalysis using 5, it was weighed in the glovebox (14.5 mg, 9.0 μmol), then dissolved in 7.5 mL of PhCl. This solution was transferred to the reactor and pressurized as described above.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to The Dow Chemical Company, Caltech, the WAVE program (D.P.), and Dow Next Generation Educator (instrumentation) for funding. We thank Dr. Sean Ewart for insightful discussions.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.9b01387.
Crystallographic data (CIF)
Additional experimental details, spectra, and crystallographic information (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Chirik P; Morris R Getting Down to Earth: The Renaissance of Catalysis with Abundant Metals. Acc. Chem. Res 2015, 48, 2495. [DOI] [PubMed] [Google Scholar]
- (2).(a) Steib AK; Kuzmina OM; Fernandez S; Flubacher D; Knochel P Efficient Chromium(II)-Catalyzed Cross-Coupling Reactions between Csp2 Centers. J. Am. Chem. Soc 2013, 135, 15346. [DOI] [PubMed] [Google Scholar]; (b) Cong X; Tang H; Zeng X Regio- and Chemoselective Kumada–Tamao–Corriu Reaction of Aryl Alkyl Ethers Catalyzed by Chromium Under Mild Conditions. J. Am. Chem. Soc 2015, 137, 14367. [DOI] [PubMed] [Google Scholar]; (c) Cong X; Fan F; Ma P; Luo M; Chen H; Zeng X Low-Valent, High-Spin Chromium-Catalyzed Cleavage of Aromatic Carbon–Nitrogen Bonds at Room Temperature: A Combined Experimental and Theoretical Study. J. Am. Chem. Soc 2017, 139, 15182. [DOI] [PubMed] [Google Scholar]; (d) Tang J; Liu P; Zeng X N-Heterocyclic carbene–chromium-catalyzed alkylative cross-coupling of benzamide derivatives with aliphatic bromides. Chem. Commun 2018, 54, 9325. [DOI] [PubMed] [Google Scholar]; (e) Chen C; Liu P; Luo M; Zeng X Kumada Arylation of Secondary Amides Enabled by Chromium Catalysis for Unsymmetric Ketone Synthesis under Mild Conditions. ACS Catal. 2018, 8, 5864 [Google Scholar]; (f) Schiwek CH; Vasilenko V; Wadepohl H; Gade LH The open d-shell enforces the active space in 3d metal catalysis: highly enantioselective chromium(ii) pincer catalysed hydrosilylation of ketones. Chem. Commun 2018, 54, 9139. [DOI] [PubMed] [Google Scholar]
- (3).(a) Wu JY; Stanzl BN; Ritter T A Strategy for the Synthesis of Well-Defined Iron Catalysts and Application to Regioselective Diene Hydrosilylation. J. Am. Chem. Soc 2010, 132, 13214. [DOI] [PubMed] [Google Scholar]; (b) García Mancheño O New Trends towards Well-Defined Low-Valent Iron Catalysts. Angew. Chem., Int. Ed 2011, 50, 2216. [DOI] [PubMed] [Google Scholar]; (c) Hazari N; Melvin PR; Beromi MM Well-defined nickel and palladium precatalysts for cross-coupling. Nat. Rev. Chem 2017, 1, 0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).(a) Monillas WH; Yap GPA; MacAdams LA; Theopold KH Binding and Activation of Small Molecules by Three-Coordinate Cr(I). J. Am. Chem. Soc 2007, 129, 8090. [DOI] [PubMed] [Google Scholar]; (b) Monillas WH; Yap GPA; Theopold KH Reactivity of a low-valent chromium dinitrogen complex. Inorg. Chim. Acta 2011, 369, 103 [Google Scholar]; (c) Akturk ES; Yap GPA; Theopold KH Mechanism-based design of labile precursors for chromium(i) chemistry. Chem. Commun 2015, 51, 15402. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) MacLeod KC; Conway JL; Patrick BO; Smith KM Exploring Chromium(III)–Alkyl Bond Homolysis with CpCr[(ArNCMe)2CH](R) Complexes. J. Am. Chem. Soc 2010, 132, 17325. [DOI] [PubMed] [Google Scholar]; (e) Emrich R; Heinemann O; Jolly PW; Kruger C; Verhovnik GPJ The Role of Metallacycles in the Chromium-Catalyzed Trimerization of Ethylene. Organometallics 1997, 16, 1511. [Google Scholar]; (f) Rozenel SS; Chomitz WA; Arnold J Chromium Complexes Supported by the Multidentate Monoanionic N2P2 Ligand: Reduction Chemistry and Reactivity with Ethylene. Organometallics 2009, 28, 6243. [Google Scholar]; (g) Albahily K; Shaikh Y; Sebastiao E; Gambarotta S; Korobkov I; Gorelsky SI Vinyl Oxidative Coupling as a Synthetic Route to Catalytically Active Monovalent Chromium. J. Am. Chem. Soc 2011, 133, 6388. [DOI] [PubMed] [Google Scholar]; (h) Wolf R; Brynda M; Ni C; Long GJ; Power PP Monomeric, Two-Coordinate, Univalent Chromium(I) Compounds: Steric Prevention of Metal–Metal Bond Formation. J. Am. Chem. Soc 2007, 129, 6076. [DOI] [PubMed] [Google Scholar]; (i) Boynton JN; Summerscales OT; Grandjean F; Long GJ; Fettinger JC; Power PP Structural and Magnetic Studies of a Quasi-Inverse Sandwich Cyclooctatetraene Complex with Two High-Spin Chromium(II) Ions Bound Anti-Facially. Organometallics 2012, 31, 8556. [Google Scholar]
- (5).(a) Bollmann A; Blann K; Dixon JT; Hess FM; Killian E; Maumela H; McGuinness DS; Morgan DH; Neveling A; Otto S; Overett M; Slawin AMZ; Wasserscheid P; Kuhlmann S Ethylene Tetramerization: A New Route to Produce 1-Octene in Exceptionally High Selectivities. J. Am. Chem. Soc 2004, 126, 14712. [DOI] [PubMed] [Google Scholar]; (b) Dixon JT; Green MJ; Hess FM; Morgan DH Advances in selective ethylene trimerisation – a critical overview. J. Organomet. Chem 2004, 689, 3641. [Google Scholar]; (c) Overett MJ; Blann K; Bollmann A; Dixon JT; Haasbroek D; Killian E; Maumela H; McGuinness DS; Morgan DH Mechanistic Investigations of the Ethylene Tetramerisation Reaction. J. Am. Chem. Soc 2005, 127, 10723. [DOI] [PubMed] [Google Scholar]; (d) Wass DF Chromium-catalysed ethene trimerisation and tetramerisation-breaking the rules in olefin oligomerisation. Dalton Trans. 2007, 816. [DOI] [PubMed] [Google Scholar]; (e) McGuinness DS Olefin Oligomerization via Metallacycles: Dimerization, Trimerization, Tetramerization, and Beyond. Chem. Rev 2011, 111, 2321. [DOI] [PubMed] [Google Scholar]; (f) Agapie T Selective ethylene oligomerization: Recent advances in chromium catalysis and mechanistic investigations. Coord. Chem. Rev 2011, 255, 861. [Google Scholar]; (g) van Leeuwen PWNM; Clément ND; Tschan MJL New processes for the selective production of 1-octene. Coord. Chem. Rev 2011, 255, 1499. [Google Scholar]
- (6).(a) Overett MJ; Blann K; Bollmann A; Dixon JT; Hess F; Killian E; Maumela H; Morgan DH; Neveling A; Otto S Ethylene trimerisation and tetramerisation catalysts with polar-substituted diphosphinoamine ligands. Chem. Commun 2005, 622. [DOI] [PubMed] [Google Scholar]; (b) Elowe PR; McCann C; Pringle PG; Spitzmesser SK; Bercaw JE Nitrogen-Linked Diphosphine Ligands with Ethers Attached to Nitrogen for Chromium-Catalyzed Ethylene Tri- and Tetramerizations. Organometallics 2006, 25, 5255. [Google Scholar]; (c) Blann K; Bollmann A; de Bod H; Dixon JT; Killian E; Nongodlwana P; Maumela MC; Maumela H; McConnell AE; Morgan DH; Overett MJ; Prétorius M; Kuhlmann S; Wasserscheid P Ethylene tetramerisation: Subtle effects exhibited by N-substituted diphosphinoamine ligands. J. Catal 2007, 249, 244. [Google Scholar]; (d) Killian E; Blann K; Bollmann A; Dixon JT; Kuhlmann S; Maumela MC; Maumela H; Morgan DH; Nongodlwana P; Overett MJ; Pretorius M; Höfener K; Wasserscheid P The use of bis(diphenylphosphino)amines with N-aryl functionalities in selective ethylene tri- and tetramerisation. J. Mol. Catal. A: Chem 2007, 270, 214. [Google Scholar]; (e) Weng Z; Teo S; Andy Hor TS Chromium(iii) catalysed ethylene tetramerization promoted by bis(phosphino)amines with an N-functionalized pendant. Dalton Trans. 2007, 3493. [DOI] [PubMed] [Google Scholar]; (f) Overett MJ; Blann K; Bollmann A; de Villiers R; Dixon JT; Killian E; Maumela MC; Maumela H; McGuinness DS; Morgan DH; Rucklidge A; Slawin AMZ Carbon-bridged diphosphine ligands for chromium-catalysed ethylene tetramerisation and trimerisation reactions. J. Mol. Catal. A: Chem 2008, 283, 114. [Google Scholar]; (g) Licciulli S; Thapa I; Albahily K; Korobkov I; Gambarotta S; Duchateau R; Chevalier R; Schuhen K Towards Selective Ethylene Tetramerization. Angew. Chem., Int. Ed 2010, 49, 9225. [DOI] [PubMed] [Google Scholar]; (h) Shaikh Y; Albahily K; Sutcliffe M; Fomitcheva V; Gambarotta S; Korobkov I; Duchateau R A Highly Selective Ethylene Tetramerization Catalyst. Angew. Chem., Int. Ed 2012, 51, 1366. [DOI] [PubMed] [Google Scholar]; (i) Newland RJ; Smith A; Smith DM; Fey N; Hanton MJ; Mansell SM Accessing Alkyl- and Alkenylcyclopentanes from Cr-Catalyzed Ethylene Oligomerization Using 2-Phosphinophosphinine Ligands. Organometallics 2018, 37, 1062. [Google Scholar]
- (7).(a) McGuinness DS; Overett M; Tooze RP; Blann K; Dixon JT; Slawin AMZ Ethylene Tri- and Tetramerization with Borate Cocatalysts: Effects on Activity, Selectivity, and Catalyst Degradation Pathways. Organometallics 2007, 26, 1108. [Google Scholar]; (b) McGuinness DS; Rucklidge AJ; Tooze RP; Slawin AMZ Cocatalyst Influence in Selective Oligomerization: Effect on Activity, Catalyst Stability, and 1-Hexene/1-Octene Selectivity in the Ethylene Trimerization and Tetramerization Reaction. Organometallics 2007, 26, 2561. [Google Scholar]
- (8).Fernelius WC; Blanch JE; Bryant BE; Terada K; Drago RS; Stille JK Chromium(III) Acetylacetonate In Inorganic Syntheses; Moeller T, Ed.; McGraw-Hill Book Company, Inc.: New York, 1957; Vol. 5, p 130. [Google Scholar]
- (9).Sydora OL; Hart RT; Eckert NA; Martinez Baez E; Clark AE; Benmore CJ A homoleptic chromium(iii) carboxylate. Dalton Trans. 2018, 47, 4790. [DOI] [PubMed] [Google Scholar]
- (10).(a) Herwig W; Zeiss H Notes: Chromium Trichloride Tetrahydrofuranate. J. Org. Chem 1958, 23, 1404. [Google Scholar]; (b) Jeon JY; Park JH; Park DS; Park SY; Lee CS; Go MJ; Lee J; Lee BY Concerning the chromium precursor CrCl3(THF)3. Inorg. Chem. Commun 2014, 44, 148. [Google Scholar]
- (11).(a) Kern RJ Tetrahydrofuran complexes of transition metal chlorides. J. Inorg. Nucl. Chem 1962, 24, 1105. [Google Scholar]; (b) Crochet A; Brog J-P; Fromm KM Mixed Metal Multinuclear Cr(III) Cage Compounds and Coordination Polymers Based on Unsubstituted Phenolate: Design, Synthesis, Mechanism, and Properties. Cryst. Growth Des 2016, 16, 189. [Google Scholar]
- (12).(a) Rabeah J; Bauer M; Baumann W; McConnell AEC; Gabrielli WF; Webb PB; Selent D; Brückner A Formation, Operation and Deactivation of Cr Catalysts in Ethylene Tetramerization Directly Assessed by Operando EPR and XAS. ACS Catal. 2013, 3, 95. [Google Scholar]; (b) Stennett TE; Haddow MF; Wass DF Avoiding MAO: Alternative Activation Methods in Selective Ethylene Oligomerization. Organometallics 2012, 31 , 6960. [Google Scholar]; (c) Do LH; Labinger JA; Bercaw JE Spectral Studies of a Cr(PNP)–MAO System for Selective Ethylene Trimerization Catalysis: Searching for the Active Species. ACS Catal. 2013, 3, 2582. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Bartlett SA; Moulin J; Tromp M; Reid G; Dent AJ; Cibin G; McGuinness DS; Evans J Activation of [CrCl3{PPh2N(iPr)PPh2}] for the selective oligomerisation of ethene: a Cr K-edge XAFS study. Catal. Sci. Technol 2016, 6, 6237. [Google Scholar]
- (13).(a) Agapie T; Schofer SJ; Labinger JA; Bercaw JE Mechanistic Studies of the Ethylene Trimerization Reaction with Chromium–Diphosphine Catalysts: Experimental Evidence for a Mechanism Involving Metallacyclic Intermediates. J. Am. Chem. Soc 2004, 126, 1304. [DOI] [PubMed] [Google Scholar]; (b) Schofer SJ; Day MW; Henling LM; Labinger JA; Bercaw JE Ethylene Trimerization Catalysts Based on Chromium Complexes with a Nitrogen-Bridged Diphosphine Ligand Having ortho-Methoxyaryl or ortho-Thiomethoxy Substituents: Well-Defined Catalyst Precursors and Investigations of the Mechanism. Organometallics 2006, 25, 2743. [Google Scholar]; (c) Vidyaratne I; Nikiforov GB; Gorelsky SI; Gambarotta S; Duchateau R; Korobkov I Isolation of a Self-Activating Ethylene Trimerization Catalyst. Angew. Chem., Int. Ed 2009, 48, 6552. [DOI] [PubMed] [Google Scholar]; (d) Albahily K; Fomitcheva V; Gambarotta S; Korobkov I; Murugesu M; Gorelsky SI Preparation and Characterization of a Reduced Chromium Complex via Vinyl Oxidative Coupling: Formation of a Self-Activating Catalyst for Selective Ethylene Trimerization. J. Am. Chem. Soc 2011, 133, 6380. [DOI] [PubMed] [Google Scholar]; (e) Monillas WH; Young JF; Yap GPA; Theopold KH A well-defined model system for the chromium-catalyzed selective oligomerization of ethylene. Dalton Trans. 2013, 42, 9198. [DOI] [PubMed] [Google Scholar]; (f) Jabri A; Mason CB; Sim Y; Gambarotta S; Burchell TJ; Duchateau R Isolation of Single-Component Trimerization and Polymerization Chromium Catalysts: The Role of the Metal Oxidation State. Angew. Chem., Int. Ed 2008, 47, 9717. [DOI] [PubMed] [Google Scholar]
- (14).Hirscher NA; Agapie T Stoichiometrically Activated Catalysts for Ethylene Tetramerization using Diphosphinoamine-Ligated Cr Tris(hydrocarbyl) Complexes. Organometallics 2017, 36, 4107. [Google Scholar]
- (15).(a) Kreisel KA; Yap GPA; Theopold KH A Chelating N-Heterocyclic Carbene Ligand in Organochromium Chemistry. Organometallics 2006, 25, 4670. [Google Scholar]; (b) Conde-Guadano S; Danopoulos AA; Pattacini R; Hanton M; Tooze RP Indenyl Functionalized N-Heterocyclic Carbene Complexes of Chromium: Syntheses, Structures, and Reactivity Studies Relevant to Ethylene Oligomerization and Polymerization. Organometallics 2012, 31, 1643. [Google Scholar]; (c) Danopoulos AA; Monakhov KY; Robert V; Braunstein P; Pattacini R; Conde-Guadaño S; Hanton M; Tooze RP Angular Distortions at Benzylic Carbons Due to Intramolecular Polarization-Induced Metal–Arene Interactions: A Case Study with Open-Shell Chromium(II) NHC Complexes. Organometallics 2013, 32, 1842. [Google Scholar]; (d) Ronellenfitsch M; Wadepohl H; Enders M Chromium Aryl Complexes with N-Donor Ligands as Catalyst Precursors for Selective Ethylene Trimerization. Organometallics 2014, 33, 5758. [Google Scholar]; (e) Ai P; Danopoulos AA; Braunstein P N-Phosphanyl- and N,N′-Diphosphanyl-Substituted N-Heterocyclic Carbene Chromium Complexes: Synthesis, Structures, and Catalytic Ethylene Oligomerization. Organometallics 2015, 34, 4109. [Google Scholar]
- (16).(a) Herwig W; Zeiss H π-Complexes of the Transition Metals. VIII. The Preparation and Reactions of Triphenylchromium(III)1. J. Am. Chem. Soc 1959, 81, 4798. [Google Scholar]; (b) Khan SI; Bau R Crystal and molecular structure of Cr(C6H5)3(OC4H8)3. Organometallics 1983, 2, 1896. [Google Scholar]
- (17).(a) Manzer LE A new chelated C,N-benzyllithium reagent and some organometallic derivatives of selected early transition metals. J. Organomet. Chem 1977, 135, C6. [Google Scholar]; (b) Manzer LE Paramagnetic organometallic compounds of the early transition metals stabilized by chelating benzyl and phenyl ligands. J. Am. Chem. Soc 1978, 100, 8068. [Google Scholar]; (c) Cotton FA; Mott GN 2-[(Dimethylamino)methyl] phenyl compounds of chromium(III) and dichromium(II) by reaction of 2-lithio-N,N-dimethylbenzylamine with dichromium(II) tetraacetate. Organometallics 1982, 1, 38 [Google Scholar]; (d)Edema JJH; Gambarotta S; Meetsma A; Spek AL Dimeric and monomeric chromium(II) and monomeric chromium(III) aryls. Crystal structure of pyramidal Mz2Cr(py) (Mz = o-Me2NCH2C6H4, py = pyridine), dimeric [(Me2NC6H4)2Cr]2, and octahedral (Me2NC6H4)3Cr. Organometallics 1992, 11, 2452 [Google Scholar]; (e) Hao S; Song J-I; Berno P; Gambarotta S Chromium(II) Organochromates. Preparation, Characterization, and Stability. Organometallics 1994, 13, 1326. [Google Scholar]; (f) Daly JJ; Sanz F; Sneeden RPA; Zeiss HH Synthesis and Structure of Tris (2′-[2-phenyl-1,3-dioxolano])-chromium(III). Helv. Chim. Acta 1974, 57, 1863 [Google Scholar]; (g) Kim EH; Lee HM; Jeong MS; Ryu JY; Lee J; Lee BY Methylaluminoxane-Free Chromium Catalytic System for Ethylene Tetramerization. ACS Omega 2017, 2, 765. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) MacLeod KC; Patrick BO; Smith KM Oxidatively Induced Reductive Elimination from a Chromium(III) Bis(aryl) Complex. Organometallics 2012, 31, 6681. [Google Scholar]; (i) McGowan KP; Abboud KA; Veige AS Trianionic NCN3− Pincer Complexes of Chromium in Four Oxidation States (CrII, CrIII, CrIV, CrV): Determination of the Active Catalyst in Selective 1-Alkene to 2-Alkene Isomerization. Organometallics 2011, 30, 4949. [Google Scholar]; (j) O’Reilly ME; Del Castillo TJ; Falkowski JM; Ramachandran V; Pati M; Correia MC; Abboud KA; Dalal NS; Richardson DE; Veige AS Autocatalytic O2 Cleavage by an OCO3− Trianionic Pincer CrIII Complex: Isolation and Characterization of the Autocatalytic Intermediate [CrIV]2(μ-O) Dimer. J. Am. Chem. Soc 2011, 133, 13661. [DOI] [PubMed] [Google Scholar]
- (18).(a) Bowen LE; Haddow MF; Orpen AG; Wass DF One electron oxidation of chromium N,N-bis(diarylphosphino)amine and bis(diarylphosphino)methane complexes relevant to ethene trimerisation and tetramerisation. Dalton Trans. 2007, 1160. [DOI] [PubMed] [Google Scholar]; (b) Rucklidge AJ; McGuinness DS; Tooze RP; Slawin AMZ; Pelletier JDA; Hanton MJ; Webb PB Ethylene Tetramerization with Cationic Chromium(I) Complexes. Organometallics 2007, 26, 2782. [Google Scholar]
- (19).(a) Bähr G; Zohm H Tri-[ω-N-diäthylamino]-tolylchrom. Angew. Chem 1963, 75, 94. [Google Scholar]; (b) Sneeden RPA Preparation In Organochromium Compounds; Sneeden RPA, Ed.; Academic Press: New York, 1975; Chapter 1, p 59. [Google Scholar]
- (20).(a) Robertson NJ; Carney MJ; Halfen JA Chromium(II) and Chromium(III) Complexes Supported by Tris(2-pyridylmethyl)-amine: Synthesis, Structures, and Reactivity. Inorg. Chem 2003, 42, 6876. [DOI] [PubMed] [Google Scholar]; (b) Rogers JS; Bu X; Bazan GC Boratabenzene Complexes of Cr(III). J. Am. Chem. Soc 2000, 122, 730. [Google Scholar]; (c) MacAdams LA; Buffone GP; Incarvito CD; Rheingold L; Theopold KH A Chromium Catalyst for the Polymerization of Ethylene as a Homogeneous Model for the Phillips Catalyst. J. Am. Chem. Soc 2005, 127, 1082. [DOI] [PubMed] [Google Scholar]; (d) Thomas BJ; Noh SK; Schulte GK; Sendlinger SC; Theopold KH Paramagnetic alkylchromium compounds as homogeneous catalysts for the polymerization of ethylene. J. Am. Chem. Soc 1991, 113, 893. [Google Scholar]
- (21).(a) Jabri A; Crewdson P; Gambarotta S; Korobkov I; Duchateau R Isolation of a Cationic Chromium(II) Species in a Catalytic System for Ethylene Tri- and Tetramerization. Organometallics 2006, 25, 715. [Google Scholar]; (b) Lifschitz AM; Hirscher NA; Lee HA ; Buss JA; Agapie T Ethylene Tetramerization Catalysis: Effects of Aluminum-Induced Isomerization of PNP to PPN Ligands. Organometallics 2017, 36, 1640. [Google Scholar]; (c) Alzamly A; Gambarotta S; Korobkov I Synthesis, Structures, and Ethylene Oligomerization Activity of Bis(phosphanylamine)pyridine Chromium/Aluminate Complexes. Organometallics 2013, 32, 7107. [Google Scholar]; (d) Alzamly A; Gambarotta S; Korobkov I Polymer-Free Ethylene Oligomerization Using a Pyridine-Based Pincer PNP-Type of Ligand. Organometallics 2013, 32, 7204. [Google Scholar]; (e) Liu S; Pattacini R; Braunstein P Reactions between an Ethylene Oligomerization Chromium(III) Precatalyst and Aluminum-Based Activators: Alkyl and Cationic Complexes with a Tridentate NPN Ligand. Organometallics 2011, 30, 3549. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


