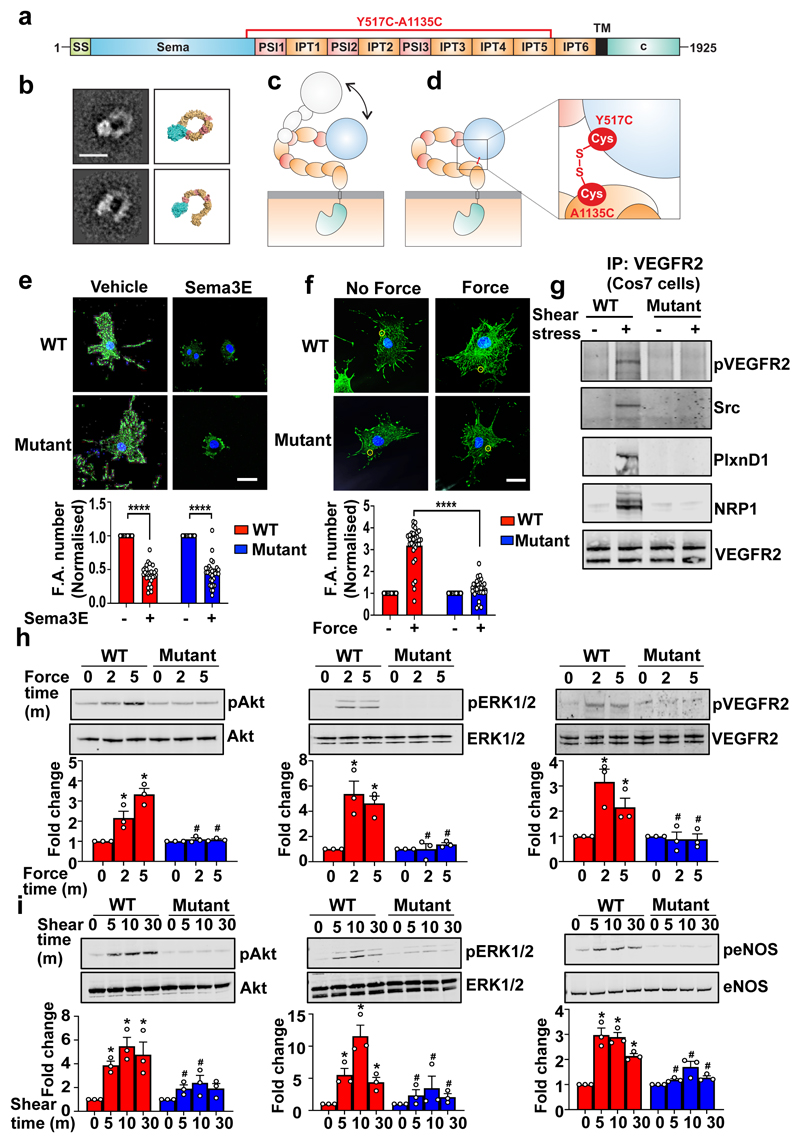
Figure 4. PlxnD1 flexion is required for mechanotransduction.
(a) Schematic domain organisation of PlxnD1 spanning amino acids 1-1925. SS, signal sequence; TM, transmembrane region; c, cytoplasmic region. (b) Representative negative stain class averages of the PlxnD1 ectodomain and corresponding structural models showing the ring-like and open conformations, scale bar 10nm. 2D class averages were obtained by classifying 1357 particles into 10 classes. (c) Model of opening the ring-like ectodomain which confers PlxnD1 mechanosensory functions. (d) Design of PlxnD1 mutant with an intramolecular disulphide bond to lock the ring-like structure. Zoom-in view shows the disulphide bond between the sema domain (domain 1) and IPT5 domain (domain 9). (e,f) ECs in which endogenous PlxnD1 was knocked down were infected with adenoviruses expressing WT or Mutant PlxnD1, treated with Sema3E for 30min or incubated with anti-PlxnD1 paramagnetic beads followed by force application (10pN; 30min). Cells were immunostained with anti-vinculin antibodies. Focal adhesion number was quantified using ImageJ; n=30 cells over either 4 (in e) or 3 (in f) biological replicates ****p<0.0001; scale bar 10 μm. (g) Cos7 cells were transfected with WT or mutant PlxnD1, NRP1 and VEGFR2 before shear stress application for 2mins and VEGFR2 was immunoprecipitated. Shear stress sensitivity was assessed by examining phospho-VEGFR2 levels, complex formation between VEGFR2 and Src and complex of PlxnD1, VEGFR2 and NRP1, n=3. (h) ECs in which endogenous PlxnD1 was knocked down were infected with adenoviruses expressing WT or mutant PlxnD1 and incubated with anti-PlxnD1 paramagnetic beads followed by force application (10pN). Phosphorylation of Akt, ERK 1/2 and VEGFR2 was determined, n=3; *p<0.05 relative to “no force” condition; #p<0.05 relative to the respective WT force time point. (i) ECs in which endogenous PlxnD1 was knocked down were infected with adenoviruses expressing WT or mutant PlxnD1 and subjected to fluid shear stress. Phosphorylation of Akt, ERK 1/2 and eNOS was determined, n=3 biological repeats; *p<0.05 relative to static condition; #p<0.05 relative to the respective WT shear time point. All data are mean±SEM. P-values were obtained by performing two-tailed Student's t test using Graphpad Prism.