Abstract
Background
Recent studies reported that impaired proximal duodenal mucosa, assessed by duodenal biopsy, could play an important role in the development of dyspeptic symptoms. The aims of this study were (a) to develop a method to measure “in vivo” duodenal and jejunal baseline impedance (BI) and (b) to assess small bowel mucosal integrity in patients with functional dyspepsia (FD) and healthy controls (HC).
Methods
We recruited 16 patients with FD and 15 HC. All subjects underwent ambulatory duodeno-jejunal manometry combined with impedance (HRM/Z), BI were determined by measuring impedance immediately after the passage of nocturnal migrating motor complex (MMC) phase IIIs.
Results
The number of MMC phase IIIs in FD was significantly lower than that in HC (2.6 ± 1.4 vs 4.8 ± 1.7, p < 0.001). The BI in patients was significantly lower than that in HC in D1(164.2 ± 59.8 Ω in FD and 243.1 ± 40.5 Ω in HC, p = 0.0061), D2 (191.2 ± 34.1 and 256.5 ± 91.4 Ω, p = 0.01), D3 (214.0 ± 76.9 and 278.1 ± 45.3 Ω, p = 0.009), D4 (270.8 ± 54.2 and 351.8 ± 50.2 Ω, p < 0.001), and J1 (312.2 ± 55.4 and 379.3 ± 38.3 Ω, p = 0.001).
Conclusions
This is the first study reporting the duodenal and jejunal BI in vivo. The results have shown significantly lowered BI in the proximal small intestine in patients with FD compared to HC. Furthermore it suggests that measurements of small bowel BI could be used as a biomarker for diagnosis and follow up of patients with FD.
Electronic supplementary material
The online version of this article (10.1007/s00535-019-01614-5) contains supplementary material, which is available to authorized users.
Keywords: Functional dyspepsia, Small bowel motility, Small bowel mucosal integrity
Introduction
Functional dyspepsia (FD) is a disorder defined by Rome IV criteria as the presence of chronic bothersome early satiety, postprandial fullness, epigastric pain or burning without any organic, systemic or metabolic disease that is likely to explain the symptoms [1]. FD is a common gastroduodenal disorder, affecting up to 15–20% of the general population [2] and is associated with significant negative impact on the quality of life [3].
Traditionally, pathophysiological factors underlying FD focused on gastric functional and/or structural abnormalities, including gastric acid hyper-secretion, impaired gastric accommodation, delayed gastric emptying and hyper-sensitivity to gastric distention and helicobacter pylori infection [4–8].
More recently, it has been proposed that another pathophysiological factor in FD can be an alteration in the duodenal mucosa [9–13]. Talley et al. reported an increased number of duodenal eosinophils and mast cells in patients with FD compared to controls [9] and suggested a role of low-grade inflammation in FD. More recent studies have reported that proximal duodenal mucosal biopsies from patients with FD showed lower transepithelial electrical resistance and increased mucosal permeability compared to those from healthy controls [10]. The authors suggested that impaired duodenal mucosal barrier function could facilitate the passage of luminal antigens through the epithelium, which may induce low-grade inflammation and would contribute to bothersome dyspeptic symptoms. Whether these mucosal abnormalities are restricted to the duodenum or they further affect the proximal small intestine is unknown.
So far, duodenal mucosal integrity has been assessed through analysis of biopsies “in vitro”. In recent years, attempts have been made to assess mucosal integrity in the esophagus “in vivo”. Intraluminal esophageal impedance is a technique to detect gastro-esophageal reflux. Impedance measurements in the absence of reflux or swallowing (baseline impedance) reflects the integrity of the esophageal mucosa [14]. Low baseline impedance in the esophagus is widely accepted as a surrogate marker of abnormal mucosal integrity [15–17].
We hypothesized that measurements of intestinal mucosal baseline impedance could be used to assess small bowel mucosal integrity “in vivo”.
The aims of this study were (1) to develop a method to measure “in vivo” duodenal and jejunal baseline impedance and (2) to assess small bowel mucosal integrity in patients with FD and healthy controls.
Methods
Subjects
We recruited a total of 16 patients (14 females and 2 males; mean age 42.1 ± 12.1 years) meeting Rome IV criteria for FD [1] and 15 healthy controls (7 females and 8 males; mean age 36.6 ± 11.5 years) at the Upper Gastrointestinal Physiology Unit of the Royal London Hospital, UK.
Patients were recruited on the basis of dyspeptic symptoms (bothersome postprandial fullness and epigastric pain) by Rome IV diagnostic questionnaire for adults. The severity of dyspeptic symptoms was scored using dyspeptic symptom score (DSS) [18]. In all FD patients, organic, systemic, or metabolic disease, likely to explain the symptoms were excluded by clinical and biochemical examination, ultrasound of the upper abdomen and esophago-gastro-duodenoscopy. Subjects with a history of abdominal surgery (other than appendicectomy), coeliac disease, or inflammatory bowel disease were excluded. Subjects had no intake of non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids or other immunosuppressive drugs in the preceding 6 months.
All healthy asymptomatic controls had both negative Helicobacter pylori infection by 13C urea breath test (Diabact UBT, Kibion, Uppsala, Sweden) and negative lactulose hydrogen breath test (LHBT).
The study protocol was approved by the ethics committee of the London – Central Research Ethics Committee (ref: 17/LO/0701) and written informed consent was obtained from all the subjects.
Ambulatory duodena-jejunal high-resolution manometry and impedance (HRM/Z)
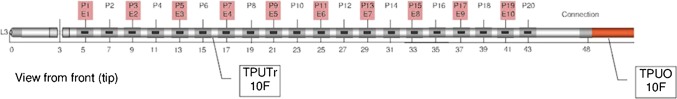
Duodeno-jejunal HRM/Z was recorded simultaneously using a dedicated ambulatory system (MMS, Version 9.2r, B.V.) and stored for subsequent display, and analysis. The HRM/Z catheter (UniSensor, Switzerland) comprises 20 pressure sensors spaced 2 cm apart and 9 pairs of impedance electrodes (Fig. 1).
Fig. 1.
High-resolution manometry combined with impedance catheter. The high-resolution manometry combined with impedance catheter (UniSensor, Switzerland) comprises 20 pressure sensors spaced 2 cm apart and 9 pairs of impedance electrodes. P pressure sensor, E electrode, TPUTr thermoplastic polyurethane transparent, TPUO thermoplastic polyurethane orange
All subjects were asked to stop proton pump inhibitors for at least 1 week prior to the study. Subjects were fasted for at least 6 h before the intubation of the HRM/Z catheter. The catheter was inserted transnasally into the stomach, and its progression was monitored using fluoroscopic screening within the limited radiation dosage (0.2–0.4 mSv for each study) [19]. When the tip of the catheter was passed through the pylorus, a balloon attached to the tip of the catheter was inflated with 5 ml of air for further propulsion. The catheter was advanced until the tip was positioned beyond the ligament of Treitz and at least three pressure sensors remained in the gastric antrum. The balloon was then deflated. Figure 2 shows the position of the catheter. Pressure and impedance sensors were distributed from the antrum to the proximal jejunum.
Fig. 2.
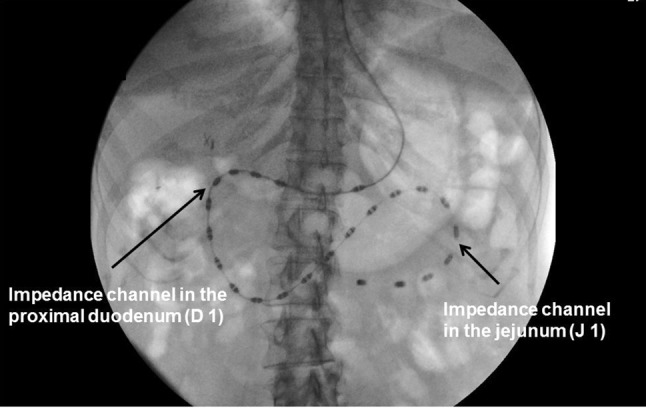
The position of catheter, pressure and impedance sensors. Pressure and impedance sensors were distributed from the antrum to the proximal jejunum
After the intubation, HRM/Z recordings were started. Subjects were then given a standard meal (630 kcal, Fat 28 g, Carbs 77 g, Protein 19 g), and rested in a sitting position for 1 h. Recordings were continued in ambulatory settings. Subjects were allowed to have only water on day 1, and they were allowed to eat their typical breakfasts on the day 2. They returned to the hospital in the morning of the day 2, and the catheter was removed. A diary was provided to record their activities including timing of meals and sleeping.
Detection of small intestinal bacterial overgrowth (SIBO) and H. pylori infection
LHBT was performed to assess SIBO. Subjects were asked to fast for 8–12 h and avoid fermentable foods such as complex carbohydrate 24 h prior to LHBT. Also, all subjects, if applicable, stopped antibiotics for at least 4 weeks and pro-motility drugs and laxatives at least one week prior to LHBT. After oral administration of 10 g of lactulose in 200 ml of water, breath samples were collected every 20 min for 120 min. A rise in hydrogen level of ≥ 20 ppm by 60 min was considered positive for SIBO [20].
Helicobacter pylori infection was assessed by 13C urea breath test. All subjects, if applicable, stopped acid suppressive medication for at least 2 weeks. After 8–12 h fasting period, breath samples were collected before and 10 min after the administration of 13C urea capsule with 200 ml water. H. pylori infection was considered to be negative if 13CO2 value was below a 2.5‰ level in the breath sample after 10 min [21, 22].
Analysis of HRM/impedance recording
The manometric parameters were analyzed both semi-automatically (quantitative) and visually (qualitative). The pressure and impedance sensors in duodenum and jejunum could be fluoroscopically identified in D1, D2, D3, D4 and J1. The nocturnal and meal periods were identified based on diary entries. Automated analysis was initially performed for the identification of duodeno-jejunal contractile events [23]. A pressure event that exceeded a threshold of 10 mmHg, for which there was no simultaneous event occurring in the other channels, was assessed by the algorithm as being the consequence of an enteric contraction.
Phase III of the migrating motor complex (MMC) was defined as the presence of a period of phasic contractions that: (1) occurred for at least 2 min; (2) recurred at a frequency of 10–12 per min in duodenum and jejunum; (3) propagated ab-orally, as indicated by at least two recording sites and (4) was subsequently followed by a period of motor quiescence (phase I) [24–26].
The following parameters in proximal duodenum (D2) were calculated: (1) Duration of phase III; (2) Peak contraction amplitude of phase III; (3) MMC cycle period. The peak contraction amplitude of phase III was taken as the peak average amplitude of MMC in each subject. The MMC cycle period was taken as a period between the onset of phase III to the next onset of phase III.
Baseline impedance measurement
In the small intestine, unlike in the esophagus, the mucosa is almost constantly covered by fluids, making it more difficult to assess the baseline mucosal impedance. We hypothesized that immediately after the passage of a phase III of the MMC, the intestinal segment is devoid of fluids and allows measurement of intestinal mucosal baseline impedance. The baseline impedance was obtained during nocturnal periods where artefacts were minimal.
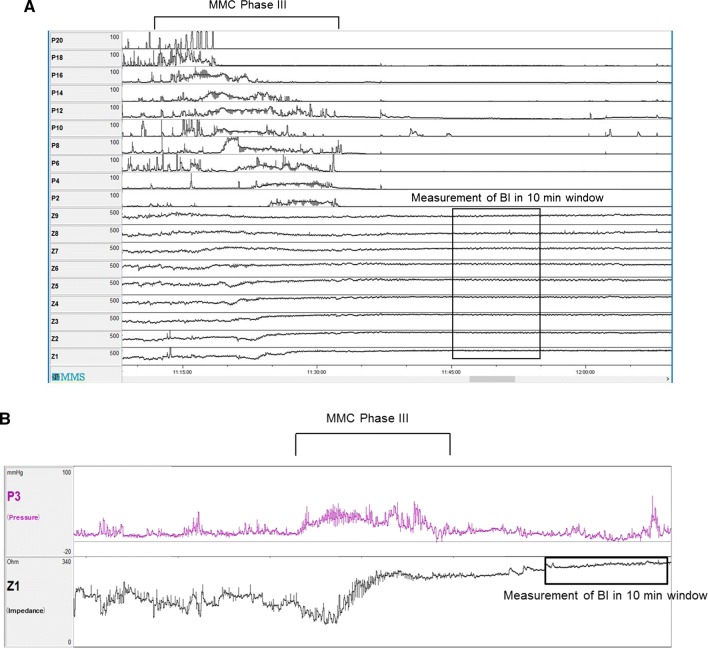
The mean baseline impedance was measured by taking an average impedance value of 10-minute time windows after the passage of MMC phase III, where a plateau in impedance was visually identified (Fig. 3a, b).
Fig. 3.
a Manometry and impedance traces at the timing of MMC pIII. MMC pIII migrating motor complex phase III, BI baseline impedance. b Example of measurement of baseline impedance. The mean baseline impedance was measured by taking an average impedance value of 10-min time windows after the passage of MMC phase III, where a plateau in impedance was visually identified. MMC migrating motor complex, P3 pressure channel 3, Z1 impedance channel 1, BI baseline impedance
Statistical analysis
All data were expressed as mean ± standard deviation (SD). Single comparisons were made with an unpaired student’s t test (parametric data) or Mann–Whitney U test (nonparametric data) wherever appropriate. Correlations were tested using the Spearman and Pearson tests wherever appropriate. Fisher’s exact test was used to test proportional differences. Significance was declared at p < 0.05. Statistical analysis was performed with Microsoft Excel 2016 or JMP Pro 14 (SAS Institute, Cary, NC, USA).
Results
All 16 patients with FD (14 females and 2 males; mean age 42.8 ± 11.8 years) and 15 healthy controls (HC) (7 females and 8 males; mean age 36.7 ± 11.5 years) completed the study. Seven patients with FD were diagnosed by Rome IV criteria as postprandial distress syndrome (PDS) and 3 were epigastric pain syndrome (EPS), 6 were overlapping PDS and EPS characteristics. Clinical characteristics of the patients were described in Table 1. There was no significant difference in age between patients and HC. The proportion of female in patients with FD was significantly higher than that in HC. Body mass index (BMI) in both groups was within the normal range. The number of H. pylori positive was 1/16 patient. Eight out of 16 patients underwent LHBT during the study periods. 1 out of 8 was positive for SIBO. Seven patients concomitant irritable bowel syndrome (IBS) symptoms. None of participants were on NSAIDs, corticosteroids or other immunosuppressive medications.
Table 1.
Clinical characteristics
| FD n = 16 | HC n = 15 | p value | |
|---|---|---|---|
| Age | 42.8 (11.8) | 36.7 (11.5) | N.S |
| Male/female | 2/14 | 8/7 | 0.023 |
| BMI | 24.8 (3.2) | 23.9 (2.9) | N.S |
| Dyspeptic symptom score | 13.5 (4.4) | 0 (0) | < 0.001 |
| H.pylori positive/negative | 1/15 | 0/15 | – |
| LHBT positive/negative | 1/7 | 0/15 | – |
Data is shown as mean ± SD
FD functional dyspepsia, HC healthy controls, BMI body mass index, H. pylori Helicobacter pylori, LHBT lactulose hydrogen breath test
Manometric parameters
The total duration of the nocturnal periods in patients with dyspepsia and control was 8.19 ± 1.6 and 8.63 ± 1.1 h (N.S.), respectively. Table 2 summarizes the parameters characterizing nocturnal duodeno-jejunal MMC phase III contractions. All subjects had at least one complete MMC cycle recorded during nocturnal period. In total, 108 nocturnal MMC phase IIIs (mean 3.92 per subject, SD 1.96) were identified. The number of MMC phase IIIs in patients was significantly lower than that in HC (2.6 ± 1.4 vs 4.8 ± 1.7, p < 0.001). The average interval of MMC cycle in FD was significantly longer than that in HC (153.4 ± 85.8 vs 81.1 ± 21.4 min, p = 0.004). There were no statistical differences in the duration of MMC phase III and the peak amplitude between the two groups (5.6 ± 2.6 vs 5.1 ± 1.7 min, N.S; 82.3 ± 16.8 vs 82.0 ± 24.7 mmHg, N.S, respectively).
Table 2.
The manometric parameters (D2)
| FD n = 16 | HC n = 15 | p value | |
|---|---|---|---|
| The number of MMC pIII | 41 | 67 | – |
| The number of MMC pIII/patient | 2.6 (1.4) | 4.8 (1.7) | < 0.001 |
| The duration of MMC pIII (min) | 3.8 (1.4) | 4.4 (1.3) | N.S |
| The average of peak amplitude (mmHg) | 82.3 (16.8) | 82.0 (24.7) | N.S |
| The average duration of MMC cycle (min) | 148.4 (82.1) | 85.8 (18.4) | 0.008 |
Data is shown as mean ± SD
FD functional dyspepsia, HC healthy controls, MMC pIII migrating motor complex phase III
Duodeno-jejunal baseline impedance
Duodeno-jejunal baseline impedance values in each segment (D1, D2, D3, D4, J1) in patients and HC were shown in Table 3 and graphically in Fig. 4. The baseline impedance increased from D1 to J1 in both FD and HC group. The baseline impedance in patients was significantly lower than that in HC in D1 (164.2 ± 59.8 Ω in FD and 243.1 ± 40.5 Ω in HC, p = 0.0061), D2 (191.2 ± 34.1 and 256.5 ± 91.4 Ω, p = 0.01), D3 (214.0 ± 76.9 and 278.1 ± 45.3 Ω, p = 0.009), D4 (270.8 ± 54.2 and 351.8 ± 50.2 Ω, p < 0.001), and J1 (312.2 ± 55.4 and 379.3 ± 38.3 Ω, p = 0.001). Also, there was no statistical difference in baseline impedance between female and male in D1 (254.1 ± 43.6 and 228.4 ± 38.9 Ω, N.S.), in D2 (275.6 ± 128.8 and 239.8 ± 42.6 Ω, N.S.), in D3 (277.7 ± 47.1 and 278.4 ± 46.8 Ω, N.S.), in D4 (356.8 ± 48.5 and 347.4 ± 54.6 Ω, N.S.), and J1 (369.1 ± 47.7 and 388.3 ± 28.1 Ω, N.S.)
Table 3.
Baseline impedance after MMC pIII
| Segment | FD n = 16 | HC n = 15 | p value |
|---|---|---|---|
| D1 | 164.2 (59.8) | 243.1 (40.5) | 0.006 |
| D2 | 191.2 (34.1) | 256.5 (91.4) | 0.01 |
| D3 | 214.0 (76.9) | 278.1 (45.3) | 0.009 |
| D4 | 270.8 (54.2) | 351.8 (50.2) | < 0.001 |
| J1 | 312.2 (55.4) | 379.3 (38.3) | 0.001 |
Data is shown as mean ± SD
MMC pIII migrating motor complex phase III, FD functional dyspepsia, HC healthy controls
Fig. 4.
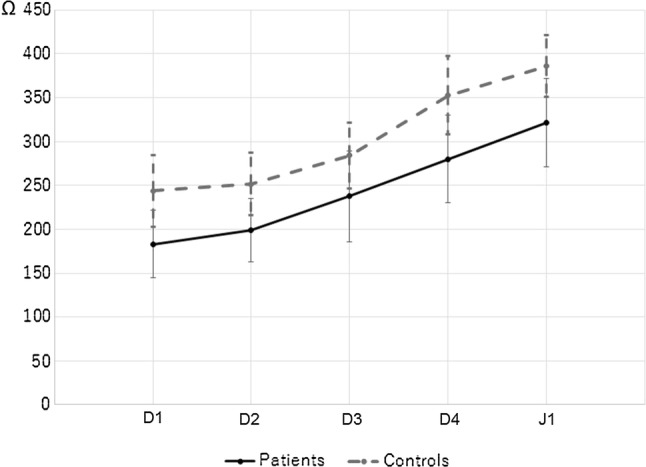
Differences in duodeno-jejunal baseline impedance in FD and HC. FD functional dyspepsia, HC healthy controls
The correlation between baseline impedance and the number of MMC phase III contractions
There were weak positive correlations between baseline impedance and the number of MMC phase III contractions in J1 (R = 0.22, p = 0.014). However, no correlations were shown in D1, D2, D3 and D4.
The correlation between baseline impedance and severity of symptoms
Severity of dyspeptic symptoms were assessed using DSS.
There were no statistical correlations between baseline impedance in the each segment and severity of symptoms (D1, R = 0.07, N.S; D2, R = 0.01, N.S; D3, R = 0.01, N.S; D4, R = 0.04, N.S; J1, R = 0.03, N.S.)
Discussion
Dyspeptic symptoms significantly impact on daily life. The causes of these symptoms, such as postprandial fullness, early satiety, epigastric discomfort/pain and burning, are not fully explained [1, 27]. However, recent studies reported that low-grade inflammation in the proximal duodenum and impaired proximal duodenal mucosal integrity, assessed by duodenal biopsy, could play an important role in the development of dyspeptic symptoms [9, 10]. Cirillo et al. reported neuronal functional abnormalities and altered ganglionic architecture in the duodenal submucous plexus in biopsies from patients with FD [28]. They suggested that low grade inflammation induced by impairment of intestinal barrier function may affect specific neuronal pathways underlying dyspeptic symptoms such as early satiety and postprandial fullness. Miwa et al. also proposed the possibility that the duodenum of patients with FD is more sensitive to noxious stimuli because of low-grade inflammation and increased mucosal permeability, and gastric motility abnormalities and gastric hypersensitive might be induced by stimulation of the duodenum [12]. In this study, we have assessed “in vivo” the integrity of duodenal and jejunal mucosa using, for the first time, measurements of baseline impedance during ambulatory duodeno-jejunal HRM-impedance monitoring.
To measure duodeno-jejunal baseline impedance, we had to simultaneously measure small intestinal motility and impedance, and identify phase III of the migrating motor complex. By doing so, we have also found that patients with FD have decreased number of phase III contractions of the MMC.
Small bowel manometry has been regarded as one of the clinical investigation tools to evaluate functional gastro-intestinal (GI) disorders. Vantrappen et al. have reported that the MMC phase III regulated by enteric nerve system is important in helping to maintain fasting aboral transit and low bacterial counts in the small intestine [26]. MMC phase III is therefore thought to be a housekeeping phenomenon clearing the gastrointestinal contents in digestive processes. In the present study, manometric finding showed the number of nocturnal MMC phase IIIs in patients with FD was significantly lower than that in HC. This result was in agreement with previous reports by Jebbink et al. [29] and Wilmer et al. [30]. They demonstrated, using ambulatory manometry technique, that MMC cycles in patients with FD occurred less frequently than in control group and suggested that this reduced incidence of MMC cycle could lead to delayed interdigestive transit then might cause dyspeptic symptoms. Also, Jacobs et al. suggested that impaired MMC phase III can cause SIBO [31]. LHBT was performed only in 8 out of 16 patients with FD. It may be therefore difficult to discuss the possible correlation between SIBO and MMC phase III. We showed that there was a weak but positive correlation between the nocturnal number of MMC phase III and baseline impedance in the proximal jejunum. This might suggest that reduced phase III leads to prolonged exposure of the jejunum to luminal contents and hence mucosal damage could occur. Further study will be needed to assess the relationship between jejunal impedance and intestinal motility.
The usage of ambulatory manometry together with impedance recordings provide the information of not only motor activity but possibly mucosal status as expressed by the baseline impedance value. To our knowledge this study has shown, for the first time, significantly lower baseline impedance from the duodenum to the proximal jejunum in patients with FD when compared to HC. The relationship between low basal impedance and symptoms is not completely clear. In the esophagus, patients with lower baseline impedance have higher esophageal sensitivity to acid exposure [32]. It is possible that similar relationship occurs in the intestine. We did not show a correlation between severity of symptoms and baseline impedance values. We should acknowledge however that perception of dyspeptic symptoms is likely to be a consequence of a complex pathophysiological cascade from intestine to central nervous system, and symptom questionnaires usually used to assess patients with FD are unlikely to be sensitive enough to detect the isolated role of impaired mucosal integrity.
Like esophageal mucosal integrity in non-erosive reflux disease, a low baseline impedance in the proximal small intestine (in the absence of endoscopic findings) could be used as a biomarker to identify patients with proximal functional GI disorders and theoretically to evaluate the outcome of treatment. However, further studies are needed to clarify whether the baseline impedance can indeed recover after treatments with acid suppression therapy [33, 34], prokinetic drugs [35] and/or acotiamide [36, 37].
In this study, a gradual increase in baseline impedance from D1 to J1 was observed in both patients with FD and HC. These impedance changes could be explained in two ways. Firstly, structural/anatomical differences of intestinal villus and tight junctions from the proximal duodenum to jejunum may affect the baseline impedance values. Secondly, duodenal mucosa could have more direct burden due to several digestive enzymes such as pepsin, hydrochloric acid as gastric juice and trypsin, amylase and lipase as pancreatic juice, which may affect the proximal duodenum most, and those chemical impacts could gradually be fading towards the jejunum.
In our patients, we found impaired mucosal integrity not only in the duodenum (as previously reported using biopsies), but also in the jejunum. FD and IBS are the two most prevalent functional gastrointestinal disorders and they might have overlapping pathophysiological mechanisms such as increased mast cell and intraepithelial lymphocyte concentrations, and increased paracellular intestinal permeability [38, 39]. It is possible, therefore that our finding of jejunal mucosal impairment in patients with FD could be due to concomitant IBS. However, our FD patients without IBS symptoms (n = 9), still had low jejunal baseline impedance compared to controls (see supplementary Table 1 and supplementary Figure 1).
The following limitations of our study are acknowledged. We did not perform microscopic assessment of mucosal changes to investigate mucosal barrier function. Our study therefore does not provide a correlation between duodenal baseline impedance and in vitro measurements of duodenal mucosa in using chambers. However, previous studies have already described that impaired duodenal mucosal integrity and permeability using biopsy sample [10] in patients with FD, and increased mucosal admittance through endoscopic technique [40] in FD compared to HC. This study did not show a significant statistical correlation between baseline impedance and severity of dyspeptic symptoms. A study using increased numbers of FD patients with wider symptom severity would further assess this possible correlation.
In conclusion, this is the first study reporting the duodenal and jejunal baseline impedance in vivo. The results have shown significantly lowered baseline impedance in the proximal small intestine in patients with FD compared to HC. These findings confirm previous “in vitro” assessments. This suggests that impaired small bowel mucosal integrity may play an important role in pathophysiology of FD. Furthermore it suggests that, as techniques are refined, measurements of small bowel baseline impedance could theoretically be used as a biomarker for diagnosis and follow up of patients with FD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- DSS
Dyspeptic symptom score
- EPS
Epigastric pain syndrome
- FD
Functional dyspepsia
- HC
Healthy controls
- HRM/Z
High-resolution manometry and impedance
- IBS
Irritable bowel syndrome
- LHBT
Lactulose hydrogen breath test
- MMC
Migrating motor complex
- NSAIDs
Non-steroidal anti-inflammatory drugs
- PDS
Postprandial distress syndrome
- SIBO
Small bowel bacterial overgrowth
- GI
Gastro-intestinal
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kenichiro Nakagawa, Email: k.nakagawa@qmul.ac.uk.
Ken Hara, Email: kenhara0704@yahoo.co.jp.
Asma Fikree, Email: asma.fikree@bartshealth.nhs.uk.
Shahab Siddiqi, Email: shahab.siddiqi@meht.nhs.uk.
Philip Woodland, Email: p.woodland@qmul.ac.uk.
Atsushi Masamune, Email: amasamune@med.tohoku.ac.jp.
Qasim Aziz, Email: q.aziz@qmul.ac.uk.
Daniel Sifrim, Email: d.sifrim@qmul.ac.uk.
Etsuro Yazaki, Email: e.yazaki@qmul.ac.uk.
References
- 1.Drossman Douglas A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features, and Rome IV. Gastroenterology. 2016;150(6):1262-1279.e2. doi: 10.1053/j.gastro.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 2.Tack J, Bisschops R, Sarnelli G. Pathophysiology and treatment of functional dyspepsia. Gastroenterology. 2004;127:1239–1255. doi: 10.1053/j.gastro.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Talley NJ. Health-related quality of life in functional dyspepsia. Aliment Pharmacol Ther. 2003;18:387–393. doi: 10.1046/j.1365-2036.2003.01706.x. [DOI] [PubMed] [Google Scholar]
- 4.Sarnelli G, Caenepeel P, Geypens B, et al. Symptoms associated with impaired gastric emptying of solids and liquids in functional dyspepsia. Am J Gastroenterol. 2003;98:783–788. doi: 10.1111/j.1572-0241.2003.07389.x. [DOI] [PubMed] [Google Scholar]
- 5.Tack J, Piessevaux H, Coulie B, et al. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346–1352. doi: 10.1016/S0016-5085(98)70012-5. [DOI] [PubMed] [Google Scholar]
- 6.Stanghellini V, Tosetti C, Paternico A, et al. Risk indicators of delayed gastric emptying of solids in patients with functional dyspepsia. Gastroenterology. 1996;110:1036–1042. doi: 10.1053/gast.1996.v110.pm8612991. [DOI] [PubMed] [Google Scholar]
- 7.Tack J, Caenepeel P, Fischler B, et al. Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology. 2001;121:526–535. doi: 10.1053/gast.2001.27180. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki H, Moayyedi P. Helicobacter pylori infection in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10:168–174. doi: 10.1038/nrgastro.2013.9. [DOI] [PubMed] [Google Scholar]
- 9.Talley NJ, Walker MM, Aro P, et al. Non-ulcer dyspepsia and duodenal eosinophilia: an adult endoscopic population-based case-control study. Clin Gastroenterol Hepatol. 2007;5:1175–1183. doi: 10.1016/j.cgh.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Vanheel H, Vicario M, Vanuytsel T, et al. Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia. Gut. 2014;63:262–271. doi: 10.1136/gutjnl-2012-303857. [DOI] [PubMed] [Google Scholar]
- 11.Oshima T, Miwa H. Functional dyspepsia—a revolution in management. Am J Gastroenterol. 2018;113:1420–1422. doi: 10.1038/s41395-018-0264-8. [DOI] [PubMed] [Google Scholar]
- 12.Miwa H, Oshima T, Tomita T, et al. Recent understanding of the pathophysiology of functional dyspepsia: role of the duodenum as the pathogenic center. J Gastroenterol. 2019;54:305–311. doi: 10.1007/s00535-019-01550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du L, Chen B, Kim JJ, et al. Micro-inflammation in functional dyspepsia: a systematic review and meta-analysis. Neurogastroenterol Motil. 2018;30:e13304. doi: 10.1111/nmo.13304. [DOI] [PubMed] [Google Scholar]
- 14.Farré R, Blondeau K, Clement D, et al. Evaluation of oesophageal mucosa integrity by the intraluminal impedance technique. Gut. 2011;60:885–892. doi: 10.1136/gut.2010.233049. [DOI] [PubMed] [Google Scholar]
- 15.Kessing BF, Bredenoord AJ, Weijenborg PW, et al. Esophageal acid exposure decreases intraluminal baseline impedance levels. Am J Gastroenterol. 2011;106:2093–2097. doi: 10.1038/ajg.2011.276. [DOI] [PubMed] [Google Scholar]
- 16.Martinucci I, de Bortoli N, Savarino E, et al. Esophageal baseline impedance levels in patients with pathophysiological characteristics of functional heartburn. Neurogastroenterol Motil. 2014;26:546–555. doi: 10.1111/nmo.12299. [DOI] [PubMed] [Google Scholar]
- 17.Woodland P, Sifrim D. Esophageal mucosal integrity in nonerosive reflux disease. J Clin Gastroenterol. 2014;48:6–12. doi: 10.1097/MCG.0b013e318299f181. [DOI] [PubMed] [Google Scholar]
- 18.Cuomo R, Sarnelli G, Grasso R, et al. Functional dyspepsia symptoms, gastric emptying and satiety provocative test: analysis of relationships. Scand J Gastroenterol. 2001;36:1030–1036. doi: 10.1080/003655201750422611. [DOI] [PubMed] [Google Scholar]
- 19.Zammit-Maempel I, Chapple CL, Leslie P. Radiation dose in videofluoroscopic swallow studies. Dysphagia. 2007;22:13–15. doi: 10.1007/s00455-006-9031-x. [DOI] [PubMed] [Google Scholar]
- 20.Rezaie A, Buresi M, Lembo A, et al. Hydrogen and methane-based breath testing in gastrointestinal disorders: the North American Consensus. Am J Gastroenterol. 2017;112:775–784. doi: 10.1038/ajg.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bessède E, Arantes V, Mégraud F, et al. Diagnosis of Helicobacter pylori infection. Helicobacter. 2017;22:3–8. doi: 10.1111/hel.12404. [DOI] [PubMed] [Google Scholar]
- 22.Ohara S, Kato M, Asaka M, et al. Studies of 13C-urea breath test for diagnosis of Helicobacter pylori infection in Japan. J Gastroenterol. 1998;33:6–13. doi: 10.1007/PL00009968. [DOI] [PubMed] [Google Scholar]
- 23.Benson MJ, Castillo FD, Wingate DL, et al. The computer as referee in the analysis of human small bowel motility. Am J Physiol Gastrointest Liver Physiol. 1993;264:645–654. doi: 10.1152/ajpgi.1993.264.4.G645. [DOI] [PubMed] [Google Scholar]
- 24.Quigley EM, Deprez PH, Hellstrom P, et al. Ambulatory intestinal manometry: a consensus report on its clinical role. Dig Dis Sci. 1997;42:2395–2400. doi: 10.1023/A:1018803819455. [DOI] [PubMed] [Google Scholar]
- 25.Camilleri M, Hasler WL, Parkman HP, et al. Measurement of gastrointestinal motility in the GI laboratory. Gastroenterology. 1998;115:747–762. doi: 10.1016/S0016-5085(98)70155-6. [DOI] [PubMed] [Google Scholar]
- 26.Vantrappen G, Janssens J, Hellemans J, et al. The interdigestive motor complex of normal subjects and patients with bacterial overgrowth of the small intestine. J Clin Invest. 1977;59:1158–1166. doi: 10.1172/JCI108740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 28.Cirillo C, Bessissow T, Desmet AS, et al. Evidence for neuronal and structural changes in submucous ganglia of patients with functional dyspepsia. Am J Gastroenterol. 2015;110:1205–1215. doi: 10.1038/ajg.2015.158. [DOI] [PubMed] [Google Scholar]
- 29.Jebbink HJ, vanBerge-Henegouwen GP, Akkermans LM, et al. Small intestinal motor abnormalities in patients with functional dyspepsia demonstrated by ambulatory manometry. Gut. 1996;38:694–700. doi: 10.1136/gut.38.5.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilmer A, Van Cutsem E, Andrioli A, et al. Ambulatory gastrojejunal manometry in severe motility-like dyspepsia: lack of correlation between dysmotility, symptoms, and gastric emptying. Gut. 1998;42:235–242. doi: 10.1136/gut.42.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobs C, Coss Adame E, Attaluri A, et al. Dysmotility and proton pump inhibitor use are independent risk factors for small intestinal bacterial and/or fungal overgrowth. Aliment Pharmacol Ther. 2013;37:1103–1111. doi: 10.1111/apt.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodland P, Al-Zinaty M, Yazaki E, et al. In vivo evaluation of acid-induced changes in oesophageal mucosa integrity and sensitivity in non-erosive reflux disease. Gut. 2013;62:1256–1261. doi: 10.1136/gutjnl-2012-302645. [DOI] [PubMed] [Google Scholar]
- 33.Blum AL, Arnold R, Stolte M, et al. Short course acid suppressive treatment for patients with functional dyspepsia: results depend on Helicobacter pylori status. The Frosch Study Group. Gut. 2000;47:473–480. doi: 10.1136/gut.47.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talley NJ, Meineche-schmidt V, Paré P, et al. Efficacy of omeprazole in functional dyspepsia: double-blind, randomized, placebo-controlled trial (the Bond and Opera studies) Aliment Pharmacol Ther. 1998;12:1055–1065. doi: 10.1046/j.1365-2036.1998.00410.x. [DOI] [PubMed] [Google Scholar]
- 35.Huang X, Lv B, Zhang S, et al. Itopride therapy for functional dyspepsia: a meta-analysis. World J Gastroenterol. 2012;18:7371–7377. doi: 10.3748/wjg.v18.i48.7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsueda K., Hongo M., Tack J., Aoki H., Saito Y., Kato H. Clinical trial: dose-dependent therapeutic efficacy of acotiamide hydrochloride (Z-338) in patients with functional dyspepsia - 100 mg t.i.d. is an optimal dosage. Neurogastroenterology & Motility. 2010;22(6):618–e173. doi: 10.1111/j.1365-2982.2009.01449.x. [DOI] [PubMed] [Google Scholar]
- 37.Ikeo K, Oshima T, Sei H, et al. Acotiamide improves stress-induced impaired gastric accommodation. Neurogastroenterol Motil. 2017;29:e12991. doi: 10.1111/nmo.12991. [DOI] [PubMed] [Google Scholar]
- 38.Martinez C, Lobo B, Pigrau M, et al. Diarrhoea-predominant irritable bowel syndrome: an organic disorder with structural abnormalities in the jejunal epithelial barrier. Gut. 2013;62:1160–1168. doi: 10.1136/gutjnl-2012-302093. [DOI] [PubMed] [Google Scholar]
- 39.Vivinus-Nebot M, Frin-Mathy G, Bzioueche H, et al. Functional bowel symptoms in quiescent inflammatory bowel diseases: role of epithelial barrier disruption and low-grade inflammation. Gut. 2014;63:744–752. doi: 10.1136/gutjnl-2012-304066. [DOI] [PubMed] [Google Scholar]
- 40.Ishigami H, Matsumura T, Kasamatsu S, et al. Endoscopy-guided evaluation of duodenal mucosal permeability in functional dyspepsia. Clin Transl Gastroenterol. 2017;8:e83–e87. doi: 10.1038/ctg.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.