ABSTRACT
Background
Iron accrued in utero is critical for fetal and infant neurocognitive development. Psychosocial stress and obesity can each suppress fetal iron accrual. Their combined effects and differences by fetal sex are not known. In an observational pregnancy cohort study in Mexico City, we investigated associations of maternal prenatal life stressors, psychological dysfunction, and prepregnancy BMI with fetal iron status at delivery.
Objectives
We hypothesized that greater maternal prenatal psychosocial stress and prepregnancy overweight and obesity are associated with lower cord blood ferritin and hemoglobin (Hb), with stronger associations in boys than girls.
Methods
Psychosocial stress in multiple domains of life stress (negative life events, perceived stress, exposure to violence) and psychological dysfunction symptoms (depression, generalized anxiety, and pregnancy-specific anxiety) were assessed with validated questionnaires during pregnancy. Prepregnancy BMI was predicted with a validated equation and categorized as normal/overweight/obese. Cord blood ferritin and Hb associations with prenatal psychosocial stress and BMI were modeled in multivariable linear regressions adjusted for maternal age, socioeconomic status, child sex, and prenatal iron supplementation. Interactions with child sex and 3-way stress-overweight/obesity-sex interactions were tested with product terms and likelihood ratio tests.
Results
In 493 dyads, median (IQR) cord blood ferritin and Hb concentrations were 185 µg/L (126–263 g/dL) and 16 g/dL (14.7–17.1 g/dL), respectively. Ferritin was lower in infants of mothers with higher prenatal perceived stress (−23%; 95% CI: −35%, −9%), violence exposure (−28%; 95% CI: −42%, −12%), anxiety symptoms (−16%; 95% CI: −27%, −4%), and obesity (−17%; 95% CI: −31%, 0.2%). Interaction models suggested sex differences and synergism between maternal stress and overweight/obesity. No associations were observed between stress or BMI and Hb.
Conclusions
Multiple prenatal psychosocial stressors and excess prepregnancy BMI were each inversely associated with fetal iron status at birth. Pregnancies and infants at elevated risk of impaired fetal iron accrual may be identifiable according to observed synergism between maternal stress and obesity and differential associations with fetal iron status by infant sex.
Keywords: developmental origins of health and disease (DOHAD), Mexico, iron deficiency, negative life events, exposure to violence, depression, anxiety, pregnancy, maternal health
Introduction
Fetal iron stores accrued during gestation are critical for early childhood development (1). An estimated 250 million children worldwide are at risk of impaired cognitive development due to inadequate iron status, other nutritional deficiencies, and inadequate nurturing care (2). Fetal iron stores that accrue in utero support fetal development and form a reserve utilized by the infant for the first 6–12 mo of life, until dietary iron supply is sufficient to meet the infant's requirements (3, 4). During mid- and late pregnancy, fetal iron accumulation is supported by increased maternal iron absorption and mobilization of tissue stores through suppression of circulating hepcidin (5). Fetal demands generally drive iron transfer, even when the mother's supply is insufficient (6). Still, when maternal iron status is very poor, or inflammation causes sequestration of circulating iron, availability of iron to the fetus can be impacted (5, 7). Insufficient fetal iron stores are associated with poor iron stores in early childhood (8–10), and impaired cognitive development in utero and during infancy (8, 11–14). Delayed umbilical cord clamping has been adopted in many settings as a strategy to improve infant iron endowment (15, 16). Still, inadequate iron availability during gestation can impair fetal neurodevelopment (12).
Several conditions are known to increase the risk of insufficient fetal iron stores, assessed in cord blood at birth. One is preterm birth, because the bulk of fetal iron stores accrue late in pregnancy (17). Additionally, when maternal iron status is severely depleted, cord blood iron becomes closely associated with maternal iron status (6, 7). Beyond these, maternal conditions including obesity (18–20), diabetes (21, 22), and infections such as malaria (23) have been associated with poorer fetal iron status, all possibly mediated by elevated circulating hepcidin (5, 19).
Maternal prenatal psychosocial stress and psychological dysfunction can similarly impair fetal iron stores. A link between prenatal psychosocial stress and fetal iron stores has been reported in preclinical studies as well as in 2 studies in humans. In rhesus monkeys, prenatally stressed pregnancies produced iron-deficient offspring with impaired immune function and abnormal emotional reactivity (24, 25). To our knowledge, only 2 studies on the topic in humans have been published. Israeli women exposed to rocket attacks during the first trimester of pregnancy had infants with lower cord blood ferritin compared with women in the same area who conceived after the rocket attacks ended (26). Additionally, in low-income women living in Wisconsin, self-reported prenatal life stressors were associated with cord blood markers of iron status (27). Psychological dysfunction, such as depression, anxiety, or posttraumatic stress disorder, can be a stressor in its own right, and can result from prior life stress or trauma (28–30). Psychological dysfunction can activate similar physiological stress mechanisms such as dysregulation of the hypothalamic-pituitary-adrenal axis (30, 31). Beyond these direct associations, psychosocial stress during pregnancy has been observed to exacerbate the effects of other toxic exposures on maternal and fetal health outcomes (32–34). The extent to which stress amplifies known associations between maternal prepregnancy overweight/obesity and fetal health outcomes is not known.
The present study was conducted in Mexico City, where anemia, obesity, and psychosocial stress are common among reproductive-age women. Although iron fortification and supplementation programs have now been in place for some time (35), anemia prevalence in women of reproductive age has persisted at >20% (36). Obesity is also highly prevalent in Mexico, with 37% of Mexican women overweight and an additional 37% obese (37). Additionally, maternal psychosocial stressors and lifetime trauma history likely reflect the prevalence of violence in Mexico (38).
In a pregnancy cohort study in Mexico City, we investigated the associations between maternal prenatal psychosocial stress and psychological dysfunction, prepregnancy overweight/obesity, and fetal iron status, and investigated sex-specific associations. We specifically included sex-specific effects because of literature reporting sex differences in links between prenatal stress and pregnancy and infant outcomes, with boys typically more vulnerable (39, 40). We hypothesized that maternal prepregnancy overweight and obesity and prenatal exposure to psychosocial stress and psychological dysfunction would each be associated with poorer fetal iron status measured at birth, and that maternal obesity and prenatal stress would interact synergistically in their associations with fetal iron status.
Methods
Participants
Participants were mother-child dyads enrolled in the Programming Research in Obesity, Growth, Environment and Social Stress (PROGRESS) prospective birth cohort study in Mexico City. Detailed information about recruitment and enrollment is published elsewhere (41, 42). Briefly, women receiving prenatal care through the Mexican Social Security System were enrolled between 2007 and 2011. Women were eligible if they were <20 wk gestation, ≥18 y of age, and planning to continue living in Mexico City. Exclusion criteria included heart or kidney disease, use of steroids or antiepilepsy drugs, and daily alcohol consumption. The study was observational; no intervention or iron or other micronutrient supplementation was provided by the study to enrolled women.
Women provided written informed consent to participate. Their infants were enrolled at birth and followed through early childhood. In total, 948 women were enrolled in the cohort and delivered a live-born infant. Protocols for the study were approved by the institutional review boards of the Icahn School of Medicine at Mount Sinai and Harvard School of Public Health as well as the Biosafety, Ethics in Research, and Research Committees of the Mexican National Institute of Public Health. Mothers were compensated for their time with small payments worth ∼15 USD for each study visit. Severe health problems detected in the course of the study procedures, including iron deficiency, were referred to the on-site physician, Dr Maria Luisa Pizano Zarate.
Iron status
Maternal blood was collected in the second and third trimesters of pregnancy and at delivery, and cord blood was collected at delivery by trained study personnel. Study personnel attendance at births was limited to regular working hours, as is common for large-scale, multiyear cohort studies. Thus, cord blood was not collected from births that occurred at night or on Sundays. These data are considered missing completely at random (MCAR), because day and time of birth are not related to any personal characteristic or exposure. Cord blood was collected from the umbilical cord as close as possible to the insertion to the placenta. Cord blood for ferritin analysis was collected in a serum tube free of anticoagulants and allowed to clot for 30 min at room temperature. Tubes were centrifuged, serum was removed to microcentrifuge tubes, and tubes stored at −20°C pending ferritin analysis. Serum ferritin was analyzed by solid-phase enzyme-labeled chemiluminescent immunometric assay on an IMMULITE 1000 (Siemens). A complete blood count (CBC) including hemoglobin (Hb) concentration was performed in maternal blood at each time point and in cord blood at birth. Cord blood aliquots were collected in tubes with EDTA to prevent clotting, for CBC and other analyses.
Prenatal stress
Domains of maternal prenatal psychosocial stress [negative life events (NLEs), perceived stress, and exposure to violence] and psychological dysfunction (symptoms of depression, generalized anxiety, pregnancy-specific anxiety) were assessed with validated Spanish-language questionnaires in the second and third trimesters of pregnancy (see below). For women with stress variables measured in the second and third trimesters, the mean value for each variable was used.
Stress variables were dichotomized using validated cutoffs when available or empirically according to the distribution of values and observed nonlinearity in the bivariate association between the continuous stress measure and fetal iron status. Dichotomous stress measures have been used previously in this cohort (33, 43) and others (44), because stress questionnaire scores typically are not normally distributed and associations between stress exposures and outcomes of interest often are not linear but display a threshold effect.
Negative life events
Women completed the Crisis in Family Systems (CRISYS) questionnaire reporting on NLEs in pregnancy (45). The questionnaire asks about events across 11 domains (financial, legal, career, relationship, home safety, neighborhood safety, medical issues–self, medical issues–others, home, prejudice, and authority) occurring during the past 6 mo. For each event, respondents rated whether the event happened, if it was ongoing, and if it was positive, negative, or neutral. The number of domains with ≥1 negative events in the past 6 mo was summed to produce an NLE domain score and then dichotomized, with 0–3 NLE domains considered low and >3 domains considered high (33, 46).
Perceived stress
Perceived stress was measured with the 4-item Perceived Stress Scale (PSS-4) (47–49). Responses were summed to generate an overall score that ranged from 0 to 15 in this sample. PSS-4 scores were dichotomized around the highest quartile of the score distribution.
Exposure to violence
Lifetime and past-1-y violence exposure by type and frequency were assessed with the Exposure to Violence (ETV) questionnaire (50). A continuous scale was developed using Rasch modeling, which gives a unidimensional score that accounts for event severity and frequency (51). The highest eighth (>85th percentile) of the distribution of the lifetime violence exposure scale was considered high exposure and compared with the lower seven-eighths in accordance with an observed nonlinear association with iron markers.
Depressive symptoms
Depressive symptoms were measured with the Edinburgh Depression Scale (EDS) (52, 53). Respondents answered 10 items referring to their experiences over the past 7 d, with possible responses: 0, rarely/none; 1, some of the time; 2, occasionally; and 3, all of the time. Two items representing positive symptoms were reverse coded and then response scales for the 10 items were summed to generate an overall score with possible values ranging from 0 to 30. Scores ≥13 were considered indicative of likely depression based on prior studies in similar populations (43, 54).
Anxiety symptoms
Symptoms of trait generalized anxiety were measured with the 10-item trait anxiety scale of the Spielberger State-Trait Anxiety Scale (STAI) (55). Responses to each item on a 4-level scale were summed to produce a total score, with higher values indicative of more anxiety symptoms.
Pregnancy-related anxiety (PrA) was measured with the Pregnancy Anxiety Scale (56). Both STAI and PrA were dichotomized around their median values in the sample.
Covariates
Maternal age and socioeconomic status were assessed at enrollment, and infant sex, birthweight, and gestational age were recorded at birth and in a postnatal maternal interview. Analyses were restricted to infants born at ≥32 wk gestational age. Socioeconomic status was assessed using the Mexican Association of Marketing Research and Public Opinion Agencies’ tool (57), and collapsed into 3 levels, as has been done previously for this cohort [e.g., Sanders et al. (58)].
Maternal dietary supplement intake over the prior month was assessed in the second and third trimesters of pregnancy with an FFQ validated in a Mexican population (59).
Weight and height were measured at the second and third trimester study visits. Prepregnancy weight was not measured directly for most women, but women were asked to self-report their prepregnancy weight. A validated algorithm developed to predict prepregnancy weight using weight(s) measured during pregnancy and other maternal characteristics was used to predict prepregnancy weight (60). Predicted prepregnancy weights were used with height measured during pregnancy to calculate prepregnancy BMI. Prepregnancy BMI (in kg/m2) was categorized as normal (BMI <25), overweight (25 to <30) or obese (≥30) (61).
Analytical methods
Data management
Anemia was defined as maternal Hb concentration <11.8 g/dL to account for the altitude in Mexico City as recommended by the WHO (62, 63). A maternal serum ferritin concentration <15 µg/L was considered indicative of iron deficiency (64). Cord blood ferritin and Hb were analyzed on a continuous scale, because cutoffs have not been established for those markers. Cord blood ferritin values were natural log-transformed prior to analysis.
Mean maternal prenatal iron supplement consumption in the second and third trimesters of pregnancy was right skewed, with most reported mean daily intakes at 0 or <40 mg/d. As such, iron supplement intake was dichotomized as: yes (nonzero mean iron intake) or no (no reported iron supplement intake).
Statistical analysis
Multivariable linear regression models were used to assess the associations between the exposures maternal prenatal stress, psychological dysfunction, and prepregnancy BMI and fetal iron status outcomes (cord blood ferritin and Hb), analyzed in separate models. Regressions were adjusted for maternal age, socioeconomic status, prenatal iron supplement intake, and child sex. Covariates were selected to remove potential bias due to confounding according to a conceptual diagram (Supplemental Figure 1).
Effect modification by infant sex of the associations of prenatal stress/psychological dysfunction and prepregnancy BMI with fetal iron status was explored in stratified models and with product terms in multivariable regressions. Three-way interactions for stress × BMI × sex were also evaluated with product terms in the regression models and tested for statistical significance using the likelihood ratio test. A P value ≤0.1 was considered statistical evidence of an interaction.
Sensitivity analyses were conducted to determine whether specific analytical decisions substantively changed the findings. To determine if the choice of empirical cutoffs drove associations between stress measures and fetal iron status markers, models for stress and psychological dysfunction measures were repeated using continuous stress scores in linear and quadratic models. To evaluate the extent to which findings related to maternal prepregnancy overweight or obesity were dependent on the prediction model used for those measures, models with maternal BMI as the exposure were rerun comparing continuous predicted prepregnancy BMI, BMI calculated with maternal self-reported prepregnancy weight, and BMI calculated with measured second-trimester weight as the independent variable.
Missing data were treated as MCAR, and main analyses were restricted to dyads with complete data, because 433 of 454 (95%) dyads with missing data were missing cord blood due to lack of 24-h attendance at deliveries by study personnel (Supplemental Figure 2). Day and time of birth are not thought to be associated with the exposure, outcome, or any other study variable. To test the MCAR assumption, full information maximum likelihood (FIML) regression models were run using the sem command with option method(mlmv). Analyses were conducted in Stata, version 15 (StataCorp) and R, version 3.5.1 (65).
Results
Data were available for 493 maternal-child dyads (Table 1). Median age of the mothers was 27.6 y. Fewer than half of mothers were of normal prepregnancy BMI (42%), whereas 39% were overweight and 19% were obese. Maternal iron and anemia status assessed in the second trimester suggested moderate prevalence of anemia: 8% of mothers had anemia (Hb <11.8 g/dL) and 16% had iron deficiency (serum ferritin <15 µg/L). Median iron supplement intake in the second and/or third trimester of pregnancy was 20 mg/d.
TABLE 1.
Characteristics of participating mothers and infants from the Programming Research in Obesity, Growth, Environment and Social Stress (PROGRESS) study in Mexico City1
| Median (IQR) or n (%) | |||
|---|---|---|---|
| Characteristic | Total | Boys | Girls |
| n | 493 | 276 (56) | 217 (44) |
| Maternal age, y | 27.7 (23.9–31.7) | 27.7 (24.2–31.9) | 27.4 (23.7–31.6) |
| SES | |||
| Low | 260 (52.7) | 140 (50.7) | 120 (55.3) |
| Medium | 178 (36.1) | 106 (38.4) | 72 (33.2) |
| Higher | 55 (11.2) | 30 (10.9) | 25 (11.5) |
| Prepregnancy BMI,2 kg/m2 | |||
| Normal (<25) | 205 (41.6) | 104 (37.7) | 101 (46.5) |
| Overweight (25 to <30) | 194 (39.4) | 120 (43.5) | 74 (34.1) |
| Obese (≥30) | 94 (19.1) | 52 (18.8) | 42 (19.4) |
| Anemic in 2T3 | 40 (8.1) | 20 (7.2) | 20 (9.2) |
| Iron deficient in 2T4 | 77 (16.0) | 42 (15.6) | 35 (16.5) |
| Iron supplement,5 mg/d | 20.0 (20.0–32.9) | 20.0 (15.0–32.9) | 21.4 (20.0–40.0) |
| Gestational age, wk | 39.0 (38.0–40.0) | 38.0 (37.0–39.0) | 39.0 (38.0–40.0) |
| Birth weight, kg | 3.1 (2.8–3.4) | 3.1 (2.9–3.4) | 3.0 (2.8–3.3) |
| Cord blood ferritin, µg/L | 185.0 (126.0–263.0) | 177.0 (115.0–251.0) | 198.0 (139.0–278.0) |
| Cord blood Hb, g/dL | 16.0 (14.7–17.1) | 16.2 (14.9–17.3) | 15.7 (14.5–16.5) |
Hb, hemoglobin; SES, socioeconomic status; 2T, second trimester of pregnancy.
Prepregnancy BMI was calculated from height measured during pregnancy, and prepregnancy weight predicted using a validated algorithm using weight(s) measured during pregnancy and other maternal characteristics (60).
Mother's iron deficiency status, deficient = serum ferritin <15 µg/L (50).
Median past 1-mo daily iron supplement intake reported on FFQs administered in the second and third trimesters of pregnancy.
Just over half of infants were male (56%). Median (IQR) birthweight was 3.1 (2.8–3.4) kg, and <10% were born <37 wk gestation. Median (IQR) cord blood ferritin and Hb concentrations were 185 (126–263) µg/L and 16 (14.7–17.1) g/dL, respectively. Ferritin was higher in girls than boys at birth, as has been reported previously (66). Maternal characteristics did not differ between those included in the analysis and those excluded because of missing fetal iron status or other data except that included mothers were about 1 y younger. Included infants had somewhat lower birthweight and were less likely to be female (Supplemental Table 1).
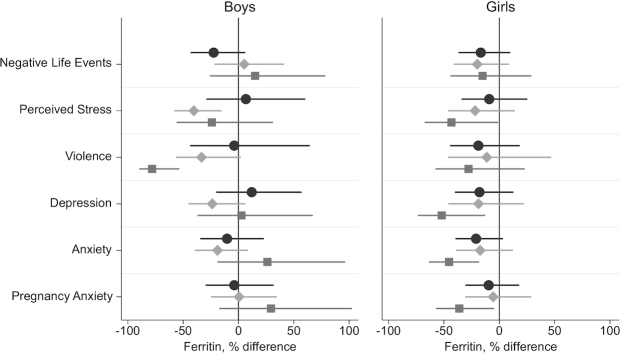
Maternal prenatal psychosocial stress in multiple domains was associated with lower cord blood ferritin (Figure 1). High prenatal NLEs were associated with lower ferritin, with larger associations in girls (18% reduction in cord blood ferritin; 95% CI: −32%, −8%). Cord blood ferritin was 23% lower (95% CI: −35%, −9%) in infants of mothers in the highest quartile of prenatal perceived stress, with similar associations observed in girls and boys. High lifetime violence exposure was associated with 28% lower cord blood ferritin (95% CI: −42%, −12%). In sex-stratified models, the magnitude of the association between the ETV score and ferritin was much larger in boys (−38%; 95% CI: −55%, −15%).
FIGURE 1.
Percentage difference in cord blood ferritin at delivery in infants of mothers with high vs. low prenatal stress (negative life events, perceived stress, and lifetime exposure to violence) or psychological dysfunction (symptoms of depression, generalized anxiety, and pregnancy anxiety), overall and stratified by infant sex. Coefficients and 95% CIs are from linear regression models with log cord-blood-ferritin as the dependent variable. Models are adjusted for maternal age, socioeconomic status, iron supplement intake, and child sex (overall models only). Associations between stress and ferritin were statistically significantly different from zero (P < 0.05) for perceived stress in boys and girls combined, for depression and anxiety in girls, and for exposure to violence overall and in boys. Interaction terms for stress by infant sex did not reach statistical significance. The stress and psychological dysfunction cutoffs and scales used were: negative life event domains ≥3 on the Crisis in Family Systems (CRISYS) questionnaire; >4th quartile (= 7) on the Perceived Stress Scale-4; >85th percentile (= 0.63) on the lifetime Exposure to Violence questionnaire; depression symptoms, ≥13 on the Edinburgh Depression Scale; anxiety symptoms, greater than the median (= 18) on the Spielberger Trait Anxiety Inventory; pregnancy anxiety symptoms, greater than the median (= 19) on the Pregnancy Anxiety Scale.
Maternal prenatal psychological dysfunction was also inversely associated with cord blood ferritin (Figure 1). For psychological dysfunction, larger associations were observed in girls than boys. In adjusted models for boys and girls combined, cord blood ferritin was 13% lower (95% CI: −25%, −2%) in infants of mothers with suspected depression (EDS score ≥13). Similarly, higher prenatal generalized anxiety symptoms (STAI score greater than the median) were associated with 16% lower cord blood ferritin (95% CI: −27%, −4%). In girls, greater maternal depression and anxiety symptoms were associated with 24% (95% CI: −40%, −5%) and 25% (95% CI: −37%, −10%) lower cord blood ferritin, respectively. Pregnancy-related anxiety (PrA) was not associated with cord blood ferritin. No stress-by-infant sex interaction term reached statistical significance at P < 0.1. None of the measures of prenatal psychosocial stress or psychological dysfunction was associated with cord blood Hb (Supplemental Table 2).
Maternal prepregnancy overweight and obesity were associated with lower cord blood ferritin in boys but not girls (Figure 2). In male infants of mothers who were overweight and obese prior to pregnancy, cord blood ferritin was lower by 23% (95% CI: −37%, −5%) and 29% (−46%, −7%), respectively, relative to infants of mothers with normal prepregnancy weight. The interaction term between categorical prepregnancy weight and infant sex was statistically significant (likelihood ratio χ2 test P value = 0.032). Prepregnancy BMI was not associated with cord blood Hb in boys or girls (not shown).
FIGURE 2.
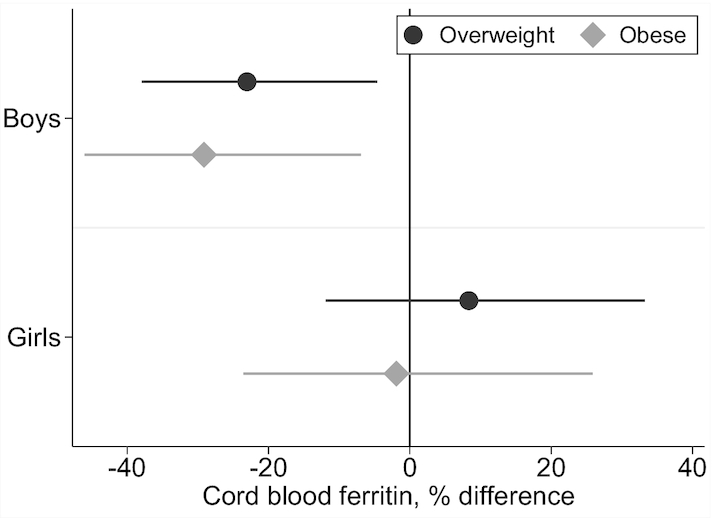
Percentage difference in cord blood ferritin at delivery in infants of mothers with prepregnancy overweight or obesity vs. normal weight. Prepregnancy BMI was calculated from height measured during pregnancy and prepregnancy weight predicted using a validated algorithm using weight(s) measured during pregnancy and other maternal characteristics (60). Coefficients and 95% CIs are from linear regression models with log cord blood ferritin as the dependent variable, indicator variables for maternal prepregnancy overweight and obesity (reference category: normal weight), and product terms for interactions between prepregnancy weight status and infant sex. Models were adjusted for maternal age, socioeconomic status, and iron supplement intake. The interaction terms between prepregnancy weight status and infant sex were statistically significant (likelihood ratio χ2P value = 0.032).
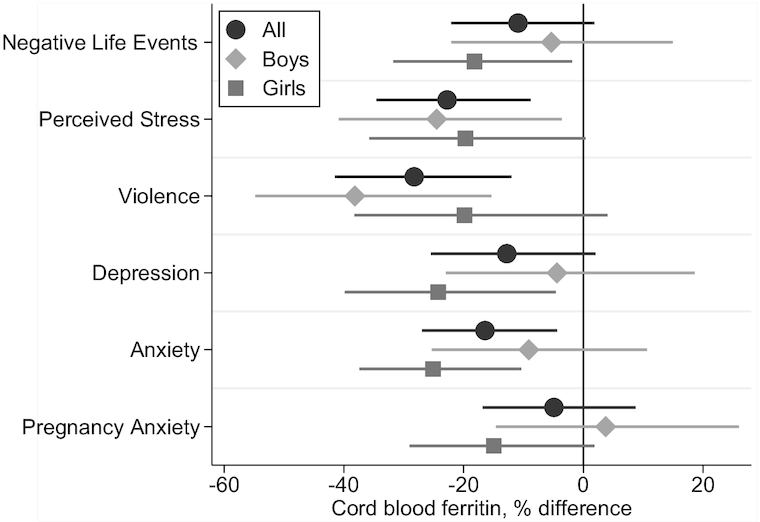
Associations of prenatal perceived stress, depression symptoms, generalized anxiety symptoms, and pregnancy anxiety with cord blood ferritin were stronger in girls of obese mothers relative to normal-weight or overweight mothers (Figure 3). In boys, maternal exposure to violence together with prepregnancy obesity was associated with cord blood ferritin >50% lower than that of infant boys of obese mothers without exposure to violence. Exposure to violence in boys of overweight mothers was suggestive of an association but the 95% CI did not exclude zero. Perceived stress was also associated with lower cord blood ferritin in boys of overweight mothers relative to normal weight. No other associations were observed between maternal stress and psychological dysfunction domains and cord blood ferritin at any maternal prepregnancy weight level. The 3-way interaction term between maternal stress variables, prepregnancy BMI, and infant sex was statistically significant for lifetime exposure to violence (P value for 3-way interaction = 0.048) and for generalized anxiety symptoms (P value = 0.0646).
FIGURE 3.
Percentage difference in cord blood ferritin at delivery in infants of mothers with high vs. low prenatal stress (negative life events, perceived stress, and lifetime exposure to violence) or psychological dysfunction (symptoms of depression, generalized anxiety, and pregnancy anxiety) by prepregnancy weight status and infant sex. Coefficients and 95% CIs are from sex-stratified linear regression models with log cord blood ferritin as the dependent variable and interaction terms between dichotomous stress and psychological dysfunction measures and categorical prepregnancy BMI. Models were adjusted for maternal age, socioeconomic status, and iron supplement intake. Stress-by-BMI interactions were significant for perceived stress in boys and girls combined (likelihood ratio χ2P value = 0.0951) and exposure to violence in boys only (P value = 0.0058). The 3-way interaction terms were statistically significant for exposure to violence (P value = 0. 048) and generalized anxiety (P value = 0.0646). The stress and psychological dysfunction cutoffs and scales used were: negative life event domains ≥3 on the Crisis in Family Systems (CRISYS) questionnaire; >4th quartile (= 7) on the Perceived Stress Scale-4; >85th percentile (= 0.63) on the lifetime Exposure to Violence questionnaire; depression symptoms, ≥13 on the Edinburgh Depression Scale; anxiety symptoms, greater than the median (= 18) on the Spielberger Trait Anxiety Inventory; pregnancy anxiety symptoms, greater than the median (= 19) on the Pregnancy Anxiety Scale. Prepregnancy BMI was calculated from height measured during pregnancy and prepregnancy weight predicted using a validated algorithm using weight(s) measured during pregnancy and other maternal characteristics (60).
Sensitivity analyses using continuous stress measures were concordant with results from models using dichotomous variables for NLE, PSS, ETV, and pregnancy anxiety, whereas models for continuous depression and anxiety symptoms demonstrated no statistically significant associations with cord blood ferritin (Supplemental Table 3). Models using BMI calculated from measured second-trimester weight or self-reported prepregnancy weight concurred with results displayed in Figure 2, which used the prediction model for prepregnancy weight (Supplemental Table 4). Potential bias resulting from missing values not being completely at random was tested in FIML models with continuous maternal independent variables using the semcommand in Stata. Results were concordant with main findings for maternal stress/psychological function (Supplemental Table 5) and BMI (Supplemental Table 6).
Discussion
In a Mexico City pregnancy cohort, maternal-reported prenatal psychosocial stress and psychological dysfunction were inversely associated with fetal iron stores at birth. These results corroborate and extend 2 prior studies that reported associations between prenatal stress and fetal iron status (26, 27). We extend those findings to an upper-middle-income country with a historically high prevalence of iron deficiency in women and children. We also report interactions between maternal stress, prepregnancy overweight and obesity, and child sex in associations with fetal iron status. Our findings suggest that maternal prenatal stress, especially when combined with excess prepregnancy BMI, can be an important risk factor for suboptimal fetal iron stores, with possible implications for fetal and early childhood neurocognitive development.
In the present study, multiple domains of maternal prenatal psychosocial stress and psychological dysfunction were associated with lower cord blood ferritin, but not with cord blood Hb. The magnitudes of the associations were large, with infants of women in the highest category of several stress exposures having mean cord blood ferritin 20–25% lower than infants of women with lower reported stress. The lack of association with cord blood Hb suggests that any relation with iron status occurred in a range that did not constrain iron availability for hematopoiesis (67). Still, low iron status can impair neurodevelopment in the absence of iron deficiency (68, 69) or anemia (22). Thus, the downward shifts in cord blood ferritin we observed with higher maternal stress, although not indicative of iron deficiency, could still affect neurodevelopment.
We also observed differences by infant sex in the stress exposures associated with fetal iron status. For most stress and psychological dysfunction domains examined, associations with fetal iron were stronger in girls than boys. The exception was maternal lifetime exposure to violence, which was very strongly associated with cord blood ferritin but in boys only. To our knowledge, prior studies of maternal psychosocial stress and fetal iron status have not investigated sex differences, but there is an extensive literature describing sex differences in associations between prenatal stress and fetal neurodevelopment (40, 70–72). Many, but not all, of those studies reported greater susceptibility in boys than girls. The reasons for sex differences in fetal susceptibility to maternal stress are largely unknown, but hormonal and genetic effects have been proposed (73).
Maternal prepregnancy BMI was also associated with lower cord blood ferritin. This concurs with prior studies (8, 18, 19), extending the finding to Mexico, where the prevalence of overweight and obesity among adult women is 73%, one of the highest in the world (37). In this study, we observed strong sex differences, with boys’ but not girls’ cord blood ferritin associated with high prepregnancy BMI. Prior studies in Mexico and elsewhere have demonstrated impairments to iron status and iron absorption in obesity (74–77). Maternal prepregnancy obesity has been linked in prior studies to impaired offspring neurocognitive development (78–80). Research is needed to investigate the contribution of dysregulated iron metabolism at the maternal–fetal interface to associations between maternal obesity and offspring neurodevelopment. Similarly, the literature describes links between maternal psychosocial stress in pregnancy and offspring neurodevelopment (81–83); additional research is needed to explore the role of fetal iron stores as a potential pathway.
In the present study, we observed effect modification by mother's BMI of associations between stress and fetal iron stores, suggesting that these 2 highly prevalent exposures might interact synergistically. Three-way interactions between prenatal stress, prepregnancy BMI, and infant sex suggested strongest associations between serum ferritin and prenatal stress exposures for girl infants of mothers who were obese prior to pregnancy. In boys, interactions between stress and prepregnancy BMI were less consistent, but maternal perceived stress in combination with prepregnancy overweight and exposure to violence in mothers with prepregnancy obesity were both strongly associated with lower cord blood ferritin. To our knowledge, this is the first study to examine the combined associations of prenatal stress and BMI with newborn iron status. Given the pervasiveness of both exposures in pregnant women and women of reproductive age, further investigation of possible synergism is warranted.
Anemia risk for women and children is classified as mild in Mexico based on current national prevalence estimates (35, 84), but the prevalence of anemia among women of reproductive age has increased again recently, attributed in part to the rising prevalence of obesity (36). Pregnant women in the present study had a relatively low prevalence of anemia (8%). Cutoffs for iron deficiency and anemia in cord blood have not been established, but few infants had cord blood ferritin values at or below concentrations previously linked to impaired newborn memory processing (3, 12). This study occurred in an urban setting with women recruited from the Mexican Social Security System (IMSS), which is linked to civil service employment and is one of the largest health insurance providers in the country. As such, participants can be expected to have regular access to good-quality health care including prenatal care, possibly resulting in a lower risk of anemia relative to the general population. Replication of our findings in other contexts with more iron deficiency will be important to elucidate the impact of the mother's iron status and iron intake on the links between maternal stress and fetal iron stores.
The study had several strengths including the large sample size and the quality and scope of data available to conduct this analysis. We had extensive prospective maternal prenatal stress and psychological dysfunction measures and cord blood ferritin and Hb measured at birth. There were limitations to the available data, including lack of inflammation markers that are independent of iron status (e.g., C-reactive protein), no gestational diabetes or dietary data, and possibly insufficient power to assess some 3-way interactions. The lack of inflammation markers in cord blood aside from ferritin warrants restraint in interpreting these findings. Still, infants were healthy; those born preterm (<32 wk) or with intrauterine growth restriction or any chronic disease or malformation were excluded from the study. Based on this and studies observing that the fetus is relatively shielded from maternal inflammation (19, 85, 86), systemic inflammation at birth is unlikely to drive observed ferritin values. Missing data for some maternal stress and psychological dysfunction measures could have introduced selection bias, because maternal life stress or mental health state could have influenced attendance at study visits or nonresponse to those questionnaires. These data were missing in a small proportion of participants, however, and FIML regression results were consistent with complete case analyses. Although conducting this analysis in the context of Mexico City extends the current literature on prenatal stress and fetal iron accumulation to a new cultural and nutritional backdrop, the moderately low prevalence of iron deficiency in the enrolled mothers limited investigation of the role of maternal iron status on the association between maternal stress exposures and fetal iron status. Finally, stress measures were self-reported, assessing mothers’ perceived stressors and symptoms, rather than being tied to a clinical diagnosis, which could have resulted in misclassification.
In conclusion, in our pregnancy cohort study in Mexico City, maternal prenatal stress and symptoms of psychological dysfunction, as well as prepregnancy overweight and obesity, were associated with lower cord blood ferritin. Associations with iron status were particularly strong for psychological dysfunction in mothers of girls and for maternal overweight and obesity in boys. Prenatal stress exposures are highly prevalent in numerous populations and settings. As such, links to fetal iron status require further research and potential clinical consideration in risk assessment for impaired fetal iron accumulation and, possibly, infant iron deficiency. Links between stress and fetal iron accumulation could also contribute to observed associations between maternal prenatal stress and fetal neurodevelopment, but that pathway requires further investigation.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the American British Cowdray Hospital in Mexico for providing research facilities.
The authors’ contributions were as follows—RKC, ROW: designed the research; RKC, MT-O, AC, LS, EO-V, RJW, MMT-R, ROW: conducted research; RKC: analyzed data, wrote the paper, and had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
Funded in part by the National Institute of Environmental Health Sciences (R01 ES013744, R01 ES014930, R01 ES021357, R24 ES028522, P30 ES023515). RKC was supported by UG3 OD023337 (National Institutes of Health Office of the Director) during the preparation of this manuscript.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figures 1 and 2 and Supplemental Tables 1–6 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations: CBC, complete blood count; EDS, Edinburgh Depression Scale; ETV, Exposure to Violence; FIML, full information maximum likelihood; Hb, hemoglobin; MCAR, missing completely at random; NLE, negative life event; PrA, pregnancy-related anxiety; PSS-4, Perceived Stress Scale; STAI, Spielberger State-Trait Anxiety Scale.
References
- 1. Black MM, Quigg AM, Hurley KM, Pepper MR. Iron deficiency and iron-deficiency anemia in the first two years of life: strategies to prevent loss of developmental potential. Nutr Rev. 2011;69(Suppl 1):S64–70. [DOI] [PubMed] [Google Scholar]
- 2. Black MM, Walker SP, Fernald LCH, Andersen CT, DiGirolamo AM, Lu C, McCoy DC, Fink G, Shawar YR, Shiffman J et al.. Early childhood development coming of age: science through the life course. Lancet. 2017;389:77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siddappa AM, Rao R, Long JD, Widness JA, Georgieff MK. The assessment of newborn iron stores at birth: a review of the literature and standards for ferritin concentrations. Neonatology. 2007;92:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rao R, Georgieff MK. Iron in fetal and neonatal nutrition. Semin Fetal Neonatal Med. 2007;12:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fisher AL, Nemeth E. Iron homeostasis during pregnancy. Am J Clin Nutr. 2017;106:1567S–74S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cao C, Fleming MD. The placenta: the forgotten essential organ of iron transport. Nutr Rev. 2016;74:421–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shao J, Lou J, Rao R, Georgieff MK, Kaciroti N, Felt BT, Zhao ZY, Lozoff B. Maternal serum ferritin concentration is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. J Nutr. 2012;142:2004–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McCarthy EK, Kenny LC, Hourihane JOB, Irvine AD, Murray DM, Kiely ME. Impact of maternal, antenatal and birth-associated factors on iron stores at birth: data from a prospective maternal-infant birth cohort. Eur J Clin Nutr. 2017;71:782–7. [DOI] [PubMed] [Google Scholar]
- 9. Georgieff MK, Wewerka SW, Nelson CA, Deregnier RA. Iron status at 9 months of infants with low iron stores at birth. J Pediatr. 2002;141:405–9. [DOI] [PubMed] [Google Scholar]
- 10. Hay G, Refsum H, Whitelaw A, Melbye EL, Haug E, Borch-Iohnsen B. Predictors of serum ferritin and serum soluble transferrin receptor in newborns and their associations with iron status during the first 2 y of life. Am J Clin Nutr. 2007;86:64–73. [DOI] [PubMed] [Google Scholar]
- 11. Tamura T, Goldenberg RL, Hou J, Johnston KE, Cliver SP, Ramey SL, Nelson KG. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J Pediatr. 2002;140:165–70. [DOI] [PubMed] [Google Scholar]
- 12. Siddappa AM, Georgieff MK, Wewerka S, Worwa C, Nelson CA, Deregnier RA. Iron deficiency alters auditory recognition memory in newborn infants of diabetic mothers. Pediatr Res. 2004;55:1034–41. [DOI] [PubMed] [Google Scholar]
- 13. East P, Delker E, Lozoff B, Delva J, Castillo M, Gahagan S. Associations among infant iron deficiency, childhood emotion and attention regulation, and adolescent problem behaviors. Child Dev. 2018;89:593–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rao R, Georgieff MK. Perinatal aspects of iron metabolism. Acta Paediatr. 2002;91:124–9. [DOI] [PubMed] [Google Scholar]
- 15. Chaparro CM, Neufeld LM, Tena Alavez G, Eguia-Liz Cedillo R, Dewey KG. Effect of timing of umbilical cord clamping on iron status in Mexican infants: a randomised controlled trial. Lancet. 2006;367:1997–2004. [DOI] [PubMed] [Google Scholar]
- 16. Committee on Obstetric Practice. Committee opinion no. 684: delayed umbilical cord clamping after birth. Obstet Gynecol. 2017;129:e5–e10. [DOI] [PubMed] [Google Scholar]
- 17. Hua Y, Kaciroti N, Jiang Y, Li X, Xu G, Richards B, Li M, Lozoff B. Inadequate iron stores in early term neonates. J Perinatol. 2018;38:1017–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Phillips AK, Roy SC, Lundberg R, Guilbert TW, Auger AP, Blohowiak SE, Coe CL, Kling PJ. Neonatal iron status is impaired by maternal obesity and excessive weight gain during pregnancy. J Perinatol. 2014;34:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dao MC, Sen S, Iyer C, Klebenov D, Meydani SN. Obesity during pregnancy and fetal iron status: is hepcidin the link?. J Perinatol. 2013;33:177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jones AD, Zhao G, Jiang YP, Zhou M, Xu G, Kaciroti N, Zhang Z, Lozoff B. Maternal obesity during pregnancy is negatively associated with maternal and neonatal iron status. Eur J Clin Nutr. 2016;70:918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lesser KB, Schoel SB, Kling PJ. Elevated zinc protoporphyrin/heme ratios in umbilical cord blood after diabetic pregnancy. J Perinatol. 2006;26:671–6. [DOI] [PubMed] [Google Scholar]
- 22. Petry CD, Eaton MA, Wobken JD, Mills MM, Johnson DE, Georgieff MK. Iron deficiency of liver, heart, and brain in newborn infants of diabetic mothers. J Pediatr. 1992;121:109–14. [DOI] [PubMed] [Google Scholar]
- 23. Pasricha S-R, Armitage AE, Prentice AM, Drakesmith H. Reducing anaemia in low income countries: control of infection is essential. BMJ. 2018;362:k3165. [DOI] [PubMed] [Google Scholar]
- 24. Rendina DN, Lubach GR, Coe CL. Gestational timing of prenatal disturbance and fetal sex determine the developmental outcomes. Neonatology. 2016;109:314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coe CL, Lubach GR, Shirtcliff EA. Maternal stress during pregnancy predisposes for iron deficiency in infant monkeys impacting innate immunity. Pediatr Res. 2007;61:520–4. [DOI] [PubMed] [Google Scholar]
- 26. Armony-Sivan R, Aviner S, Cojocaru L, Fytlovitch S, Ben-Alon D, Eliassy A, Babkoff H, Lozoff B, Anteby E. Prenatal maternal stress predicts cord-blood ferritin concentration. J Perinat Med. 2013;41:259–65. [DOI] [PubMed] [Google Scholar]
- 27. Rendina DN, Blohowiak SE, Coe CL, Kling PJ. Maternal perceived stress during pregnancy increases risk for low neonatal iron at delivery and depletion of storage iron at one year. J Pediatr. 2018;200:166–73. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rich-Edwards JW, James-Todd T, Mohllajee A, Kleinman K, Burke A, Gillman MW, Wright RJ. Lifetime maternal experiences of abuse and risk of pre-natal depression in two demographically distinct populations in Boston. Int J Epidemiol. 2011;40:375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR, Giles WH. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256:174–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–7. [DOI] [PubMed] [Google Scholar]
- 31. O'Donnell K, O'Connor TG, Glover V. Prenatal stress and neurodevelopment of the child: focus on the HPA axis and role of the placenta. Dev Neurosci. 2009;31:285–92. [DOI] [PubMed] [Google Scholar]
- 32. Tamayo YOM, Tellez-Rojo MM, Trejo-Valdivia B, Schnaas L, Osorio-Valencia E, Coull B, Bellinger D, Wright RJ, Wright RO. Maternal stress modifies the effect of exposure to lead during pregnancy and 24-month-old children's neurodevelopment. Environ Int. 2017;98:191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rosa MJ, Just AC, Kloog I, Pantic I, Schnaas L, Lee A, Bose S, Chiu YM, Hsu HL, Coull B et al.. Prenatal particulate matter exposure and wheeze in Mexican children: effect modification by prenatal psychosocial stress. Ann Allergy Asthma Immunol. 2017;119:232–7. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee A, Leon Hsu HH, Mathilda Chiu YH, Bose S, Rosa MJ, Kloog I, Wilson A, Schwartz J, Cohen S, Coull BA et al.. Prenatal fine particulate exposure and early childhood asthma: effect of maternal stress and fetal sex. J Allergy Clin Immunol. 2018;141:1880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mujica-Coopman MF, Brito A, Lopez de Romana D, Rios-Castillo I, Coris H, Olivares M. Prevalence of anemia in Latin America and the Caribbean. Food Nutr Bull. 2015;36:S119–28. [DOI] [PubMed] [Google Scholar]
- 36. Shamah-Levy T, Mejia-Rodriguez F, Mendez Gomez-Humaran I, De la Cruz-Gongora V, Mundo-Rosas V, Villalpando-Hernandez S. [Trend in the prevalence of anemia in Mexican women of childbearing age from 2006–2016. Ensanut MC 2016] Salud Publica Mex. 2018;60:301–8. Spanish. [DOI] [PubMed] [Google Scholar]
- 37. Barquera S, Campos-Nonato I, Hernandez-Barrera L, Flores M, Durazo-Arvizu R, Kanter R, Rivera JA. Obesity and central adiposity in Mexican adults: results from the Mexican National Health and Nutrition Survey 2006. Salud Publica Mex. 2009;51(Suppl 4):S595–603. [DOI] [PubMed] [Google Scholar]
- 38. Canudas-Romo V, Aburto JM, García-Guerrero VM, Beltrán-Sánchez H. Mexico's epidemic of violence and its public health significance on average length of life. J Epidemiol Community Health. 2017;71:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bale TL, Epperson CN. Sex differences and stress across the lifespan. Nat Neurosci. 2015;18:1413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. DiPietro JA, Voegtline KM. The gestational foundation of sex differences in development and vulnerability. Neuroscience. 2017;342:4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Braun JM, Wright RJ, Just AC, Power MC, Tamayo YOM, Schnaas L, Hu H, Wright RO, Tellez-Rojo MM. Relationships between lead biomarkers and diurnal salivary cortisol indices in pregnant women from Mexico City: a cross-sectional study. Environ Health. 2014;13:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Burris HH, Braun JM, Byun H-M, Tarantini L, Mercado A, Wright RJ, Schnaas L, Baccarelli AA, Wright RO, Tellez-Rojo MM. Association between birth weight and DNA methylation of IGF2, glucocorticoid receptor and repetitive elements LINE-1 and Alu. Epigenomics. 2013;5:271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Munoz-Rocha TV, Tamayo YOM, Romero M, Pantic I, Schnaas L, Bellinger D, Claus-Henn B, Wright R, Wright RO, Tellez-Rojo MM. Prenatal co-exposure to manganese and depression and 24-months neurodevelopment. Neurotoxicology. 2018;64:134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee AG, Chiu YM, Rosa MJ, Cohen S, Coull BA, Wright RO, Morgan WJ, Wright RJ. Association of prenatal and early childhood stress with reduced lung function in 7-year-olds. Ann Allergy Asthma Immunol. 2017;119:153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shalowitz MU, Berry CA, Rasinski KA, Dannhausen-Brun CA. A new measure of contemporary life stress: development, validation, and reliability of the CRISYS. Health Serv Res. 1998;33:1381–402. [PMC free article] [PubMed] [Google Scholar]
- 46. Chiu YHM, Coull BA, Cohen S, Wooley A, Wright RJ. Prenatal and postnatal maternal stress and wheeze in urban children: effect of maternal sensitization. Am J Respir Crit Care Med. 2012;186:147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. [PubMed] [Google Scholar]
- 48. Lee EH. Review of the psychometric evidence of the perceived stress scale. Asian Nurs Res (Korean Soc Nurs Sci). 2012;6:121–7. [DOI] [PubMed] [Google Scholar]
- 49. Vallejo MA, Vallejo-Slocker L, Fernandez-Abascal EG, Mananes G. Determining factors for stress perception assessed with the Perceived Stress Scale (PSS-4) in Spanish and other European samples. Front Psychol. 2018;9:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Selner-O'Hagan MB, Kindlon DJ, Buka SL, Raudenbush SW, Earls FJ. Assessing exposure to violence in urban youth. J Child Psychol Psychiat. 1998;39:215–24. [PubMed] [Google Scholar]
- 51. Suglia SF, Ryan L, Wright RJ. Creation of a community violence exposure scale: accounting for what, who, where, and how often. J Trauma Stress. 2008;21:479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–6. [DOI] [PubMed] [Google Scholar]
- 53. Flom JD, Chiu YM, Tamayo-Ortiz M, Schnaas L, Curtin PC, Wright RJ, Wright RO, Tellez-Rojo MM, Rosa MJ. Subconstructs of the Edinburgh Postpartum Depression Scale in a postpartum sample in Mexico City. J Affect Disord. 2018;238:142–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Alvarado R, Guajardo V, Rojas G, Jadresic E. Validación de la Escala de Edimburgo para embarazadas. Santiago, Chile: Universidad de Chile Facultad de Medicina; 2012. [Google Scholar]
- 55. Spielberger CD. Manual for the State-Trait Anxiety Inventory STAI (form Y). Consulting Psychologists Press, Inc; 1983. [Google Scholar]
- 56. DiPietro JA, Ghera MM, Costigan K, Hawkins M. Measuring the ups and downs of pregnancy stress. J Psychosom Obstet Gynaecol. 2004;25:189–201. [DOI] [PubMed] [Google Scholar]
- 57. Carrasco A. The AMAI system of classifying households by socio-economic level. Amsterdam (the Netherlands): ESOMAR; 2002. [Google Scholar]
- 58. Sanders AP, Svensson K, Gennings C, Burris HH, Oken E, Amarasiriwardena C, Basnet P, Pizano-Zarate ML, Schnaas L, Tamayo-Ortiz M et al.. Prenatal lead exposure modifies the effect of shorter gestation on increased blood pressure in children. Environ Int. 2018;120:464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hernandez-Avila M, Romieu I, Parra S, Hernandez-Avila J, Madrigal H, Willett W. Validity and reproducibility of a food frequency questionnaire to assess dietary intake of women living in Mexico City. Salud Publica Mex. 1998;40:133–40. [DOI] [PubMed] [Google Scholar]
- 60. Thomas DM, Oken E, Rifas-Shiman SL, Tellez-Rojo M, Just A, Svensson K, Deierlein AL, Chandler-Laney PC, Miller RC, McNamara C et al.. Do women know their prepregnancy weight? Obesity (Silver Spring). 2019;27:1161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Centers for Disease Control and Prevention. About adult BMI. CDC, 2017, [Internet]. [cited February 6, 2019]. Available from: https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html. [Google Scholar]
- 62. Sullivan KM, Mei Z, Grummer-Strawn L, Parvanta I. Haemoglobin adjustments to define anaemia. Trop Med Int Health. 2008;13:1267–71. [DOI] [PubMed] [Google Scholar]
- 63. World Health Organization. Nutritional anaemias: tools for effective prevention and control. Geneva: WHO; 2017. [Google Scholar]
- 64. World Health Organization. Iron deficiency anaemia: assessment, prevention and control: Geneva: WHO; 2001. [Google Scholar]
- 65. R Core Team. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2017. [Google Scholar]
- 66. Tamura T, Hou J, Goldenberg RL, Johnston KE, Cliver SP. Gender difference in cord serum ferritin concentrations. Biol Neonate. 1999;75:343–9. [DOI] [PubMed] [Google Scholar]
- 67. Georgieff MK. Iron assessment to protect the developing brain. Am J Clin Nutr. 2017;106:1588S–93S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zamora TG, Guiang SF 3rd, Widness JA, Georgieff MK. Iron is prioritized to red blood cells over the brain in phlebotomized anemic newborn lambs. Pediatr Res. 2016;79:922–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rao R, Ennis K, Lubach GR, Lock EF, Georgieff MK, Coe CL. Metabolomic analysis of CSF indicates brain metabolic impairment precedes hematological indices of anemia in the iron-deficient infant monkey. Nutr Neurosci. 2018;21:40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fineberg AM, Ellman LM, Schaefer CA, Maxwell SD, Shen L, Chaudhury NH, Cook AL, Bresnahan MA, Susser ES, Brown AS. Fetal exposure to maternal stress and risk for schizophrenia spectrum disorders among offspring: differential influences of fetal sex. Psychiatry Res. 2016;236:91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhu P, Hao JH, Tao RX, Huang K, Jiang XM, Zhu YD, Tao FB. Sex-specific and time-dependent effects of prenatal stress on the early behavioral symptoms of ADHD: a longitudinal study in China. Eur Child Adolesc Psychiatry. 2015;24:1139–47. [DOI] [PubMed] [Google Scholar]
- 72. Van den Bergh BRH, van den Heuvel MI, Lahti M, Braeken M, de Rooij SR, Entringer S, Hoyer D, Roseboom T, Raikkonen K, King S et al.. Prenatal developmental origins of behavior and mental health: the influence of maternal stress in pregnancy. Neurosci Biobehav Rev. 2017, [Internet]. doi: 10.1016/j.neubiorev.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 73. Arnold AP. Sex chromosomes and brain gender. Nat Rev Neurosci. 2004;5:701–8. [DOI] [PubMed] [Google Scholar]
- 74. Jones AD, Mundo-Rosas V, Cantoral A, Levy TS. Household food insecurity in Mexico is associated with the co-occurrence of overweight and anemia among women of reproductive age, but not female adolescents. Matern Child Nutr. 2017;13:e12396. doi: 10.1111/mcn.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kroker-Lobos MF, Pedroza-Tobias A, Pedraza LS, Rivera JA. The double burden of undernutrition and excess body weight in Mexico. Am J Clin Nutr. 2014;100:1652S–8S. [DOI] [PubMed] [Google Scholar]
- 76. Cepeda-Lopez AC, Osendarp SJM, Melse-Boonstra A, Aeberli I, Gonzalez-Salazar F, Feskens E, Villalpando S, Zimmermann MB. Sharply higher rates of iron deficiency in obese Mexican women and children are predicted by obesity-related inflammation rather than by differences in dietary iron intake. Am J Clin Nutr. 2011;93:975–83. [DOI] [PubMed] [Google Scholar]
- 77. Cepeda-Lopez AC, Aeberli I, Zimmermann MB. Does obesity increase risk for iron deficiency? A review of the literature and the potential mechanisms. Int J Vitam Nutr Res. 2010;80:263–70. [DOI] [PubMed] [Google Scholar]
- 78. Girchenko P, Lahti-Pulkkinen M, Lahti J, Pesonen AK, Hamalainen E, Villa PM, Kajantie E, Laivuori H, Reynolds RM, Raikkonen K. Neonatal regulatory behavior problems are predicted by maternal early pregnancy overweight and obesity: findings from the prospective PREDO Study. Pediatr Res. 2018;84:875–81. [DOI] [PubMed] [Google Scholar]
- 79. Girchenko P, Tuovinen S, Lahti-Pulkkinen M, Lahti J, Savolainen K, Heinonen K, Pyhala R, Reynolds RM, Hamalainen E, Villa PM et al.. Maternal early pregnancy obesity and related pregnancy and pre-pregnancy disorders: associations with child developmental milestones in the prospective PREDO Study. Int J Obes (Lond). 2018;42:995–1007. [DOI] [PubMed] [Google Scholar]
- 80. Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL, Hertz-Picciotto I. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 2012;129:e1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Campbell RK, Devick KL, Coull BA, Cowell W, Askowitz T, Goldson B, Wright RO, Wright RJ. Prenatal cortisol modifies the association between maternal trauma history and child cognitive development in a sex-specific manner in an urban pregnancy cohort. Stress. 2019;22:228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bosquet Enlow M, Devick KL, Brunst KJ, Lipton LR, Coull BA, Wright RJ. Maternal lifetime trauma exposure, prenatal cortisol, and infant negative affectivity. Infancy. 2017;22:492–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lang AJ, Gartstein MA, Rodgers CS, Lebeck MM. The impact of maternal childhood abuse on parenting and infant temperament. J Child Adolesc Psychiatr Nurs. 2010;23:100–10. [DOI] [PubMed] [Google Scholar]
- 84. Rivera JA, Sotres-Alvarez D, Habicht J-P, Shamah T, Villalpando S. Impact of the Mexican Program for Education, Health, and Nutrition (Progresa) on rates of growth and anemia in infants and young children: a randomized effectiveness study. JAMA. 2004;291:2563–70. [DOI] [PubMed] [Google Scholar]
- 85. Phillips AK, Roy SC, Lundberg R, Guilbert TW, Auger AP, Blohowiak SE, Coe CL, Kling PJ. Neonatal iron status is impaired by maternal obesity and excessive weight gain during pregnancy. J Perinatol. 2014;34:513–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Korlesky C, Kling PJ, Pham DQD, Ovasapyan AA, Leyns CEG, Weber MB, Coe CL. Cord blood erythropoietin and hepcidin reflect lower newborn iron stores due to maternal obesity during pregnancy. Am J Perinatol. 2019;36:511–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.