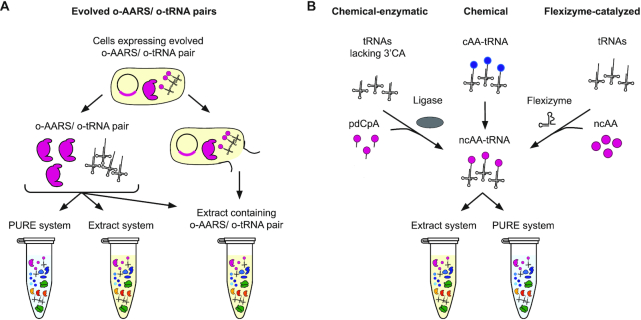
Figure 3.
tRNA aminoacylation methods for non-canonical amino acid incorporation. Methods for tRNA aminoacylation may be divided into two categories – those which leverage engineered orthogonal variants of the protein aminoacyl-tRNA synthetases (o-AARS) used by organisms to charge tRNAs in cells, and those which bypass this system via alternative routes. Systems using an o-AARS/o-tRNA pair (left) follow one of two methodologies. In the first, the o-AARS and o-tRNA are individually purified and may be added at any desired concentration to either a PURE reaction or an extract-based reaction. If fewer purification steps are desired, the o-AARS/o-tRNA pair maybe expressed in cells from which an extract is directly prepared, alleviating the need to supplement it in the reaction, but ceding some control of reaction conditions. Alternative routes (right) are often used for monomers which do not have engineered o-AARS variants available. The first involves T4 ligase-mediated ligation of an aminoacylated pdCpA to a truncated tRNA (right-left). The second avoids the challenging ligation step by chemically modifying a monomer which is already aminoacylated to a tRNA by a native AARS (right-center). Lastly, artificial ribozymes called Flexizymes may be utilized to aminoacylate tRNAs with a wide range of non-canonical amino acids and other monomers (right-right). Once obtained and purified, these aminoacylated tRNAs may be used readily in a PURE or extract-based method for translation. Strain engineering or selective depletion can be used to modify the content of translational components in the extract.