Abstract
Objective
Visceral artery pseudoaneurysms (VAPA) are associated with a high morbidity and mortality, but sometimes are missed in initial computed tomography (CT) examinations. The aims of this study were to determine the frequency and causes of misdiagnoses of VAPA with CT.
Materials and Methods
We retrospectively identified 77 patients with VAPA in our database who underwent contrast-enhanced CT. The frequency of delayed diagnosis was determined and the reasons were noted. We identified the etiology of VAPA, measured size, and noted the affected vessels.
Results
Forty-five of the 77 patients (58 %) had a delayed diagnosis of VAPA. There was no difference in the rate of missed VAPA in symptomatic compared to asymptomatic patients (p = 0.255). The majority of VAPA were associated with previous surgery or interventions (n = 48/62 %). The major affected vessel was the hepatic (n = 31) followed by the splenic artery (n = 17). The main reasons for misdiagnosis were a missed arterial phase in CT (n = 16/36 %), artifacts masking the aneurysm (n = 9/20 %), overlooked pseudoaneurysm (n = 19/42 %), and misinterpretation by attending radiologists (n = 1/2 %). Missed VAPA were smaller (median 8 mm) than those VAPA that were initially diagnosed (median 13 mm, p < 0.01), but occurred with a similar frequency in larger and smaller visceral arteries (p = 0.601).
Conclusions
Our study showed that 58 % of VAPA were diagnosed with delay, with the following four reasons for misdiagnosis: Lack of an arterial contrast phase in CT, no techniques for artifact reduction, and lack of awareness of the radiologists. Avoiding delayed diagnosis will most probably improve outcome of patients with VAPA.
Keywords: Visceral artery, Pseudoaneurysm, Computed tomography, Endovascular procedures
1. Introduction
Visceral artery aneurysms are aneurysms affecting the celiac, superior or inferior mesenteric arteries and their branches. In true visceral artery aneurysms, all three layers of the arterial wall are bulging, whereas in visceral artery pseudoaneurysms (VAPA), there is a tear in the vessel wall and only the adventitial layer of the vascular wall is bulging [1].VAPA are typically the result of inflammation, peptic ulcer disease, dissection or trauma including iatrogenic causes such as surgery or interventional procedures [[2], [3], [4], [5]]. Another rare cause of pseudoaneurysm formation is segmental arterial mediolysis (SAM), where a tear separates the outer medial muscle from the adventitia [[6], [7], [8]]. Mainly because of more widespread and progressively extensive hepatobiliary surgery and percutaneous interventions, the incidence of VAPA has been suspected to increase [9,10]. Moreover, the increasingly unrestricted use of cross-sectional abdominal imaging such as computed tomography (CT) and magnetic resonance (MR) imaging has led to a higher detection rate of visceral aneurysm in recent years [[11], [12], [13]]. Still, VAPA are considered to be underdiagnosed, and patients often present to the hospital only when complications occur [14,15].
The most common complication of VAPA is rupture and hemorrhage, with potentially devastating consequences for the patient both in terms of morbidity and mortality [9,11,14]. Recent studies reported a rupture rate of pseudoaneurysms of 76,3 %, with a high mortality rate, up to 21 % for visceral aneurysm [11,14]. Thus, early recognition of VAPA seems to be essential for early and appropriate treatment.
According to our clinical experience, VAPA continue to be missed despite of the extensive use of CT imaging. Thus, the aims of this study were first, to determine the frequency of misdiagnoses of VAPA in our department and second, to determine the reasons for these misdiagnoses.
2. Material and methods
2.1. Patient population
In this retrospective study, we searched in our electronic database system in the years from January 2001 to May 2018 for the terms “pseudoaneurysm”, “aneurysm of visceral artery”, “false aneurysm”, and “aneurysma spurium” in all radiological reports, yielding a total of 617 patients who underwent a contrast-enhanced CT examination. Pseudoaneurysms of the abdominal aorta and outflow vessels (n = 228), thoracic aorta, and of other thoracic (n = 288) and renal vessels (n = 15) were excluded. Nine patients with VAPA’s were excluded because the final diagnosis was a true visceral aneurysm. The remaining 77 patients (20 female, 57 male, mean age 57 ± 15 years) with VAPA were included in this study (Table 1).
Table 1.
Patient characteristics.
| Patients | 77 |
|---|---|
| Gender | 57 male / 20 female |
| Age (years) | Mean 56.5 ± 10.0 (range 16–89) |
| Co-Morbidities | 56 (73 %) |
| Hypertension | 38 (49 %) |
| Diabetes | 8 (10 %) |
| Smoker | 35 (45 %) |
| Nephropathy | 20 (26 %) (9 acute, 11 chronic) |
When available, the diagnosis of VAPA was made on the basis of pathologic studies or autopsy data. When pathologic specimens were not available, the diagnosis was based on patient history including recurrent or acute pancreatitis, trauma, previous abdominal interventions or surgery in conjunction with repetitive radiologic examinations, as previously shown [14]. Digital subtraction angiography (DSA) was available in 70 of the 77 patients (91 %); the remaining 7 patients had repetitive CT follow-up. Six patients had an additional MR imaging examination during follow-up.
When the final diagnosis of VAPA was confirmed, all previous imaging studies (especially the initial CT), were re-evaluated by an experienced radiologist who noted the presence of a VAPA. If a pseudoaneurysm was present we reviewed the written report and noted if the correct diagnosis was made in initial CT.
This retrospective study had institutional board approval, written consent requirement was waived.
2.2. Data analysis
We reviewed the clinical data and electronic medical records of all 77 patients. The reviewed variables included age, sex, hypertension, diabetes, smoking, renal failure, symptoms and clinical presentation at the time of initial CT, previous surgery and interventions, and treatment modality. Patient outcome was investigated at the end of hospitalization and for a six months follow up, from medical reports and from imaging in our electronic medical system. Radiological reports were reviewed and the reasons (indications) for referral to CT were noted. All CT, DSA and MR images were analyzed by two radiologists in consensus. The two readers noted the location and size of VAPA. The latter was determined as maximum diameter (measured with an electronic caliper) in initial CT. Causes for missing the diagnosis of VAPA in initial CT were categorized as follows:
1. Missing contrast media phase;
2. Artifacts masking the pseudoaneurysm;
3. Overlooked pseudoaneurysms not recognized by the attending radiologist; and
4. False interpretation of the CT imaging findings.
3. Results
Two patients (3 %) had two VAPAs and one patient presented with three VAPAs in different visceral arteries (total number of VAPA in the 77 patients, n = 81).
Of the 77 patients with VAPA, 32 (42 %) were diagnosed correctly at first CT, meaning that the VAPA was mentioned in the report of the attending radiologist in the initial CT examination. In the remaining 45 patients (58 %), the VAPA was not initially diagnosed (i.e., not mentioned in the first report) and thus was considered “missed” (Fig. 1). In all of the three patients with more than one VAPA, at least one pseudoaneurysm was missed in the initial radiological report.
Fig. 1.
Study flowchart.
3.1. Etiology of VAPA
In the majority of cases (n = 48, 62 %), the etiology of VAPA was a foregoing medical procedure (either surgery or intervention). The majority of patients in this subgroup (n = 19, 25 %) underwent pancreas surgery (mostly Whipple procedures (n = 10, 13 %) or others (n = 9, 12 %)), followed by hepatobiliary surgery (transplantation, hemihepatectomy or cholecystectomy, n = 12, 16 %). In four cases (5 %) surgery involved the gastrointestinal tract (including gastrectomy and small bowel resection) and eight patients (10 %) had other surgery such as renal transplantation and Y-grafting of the aorta. Eight patients (10 %) underwent minimally invasive interventions such as percutaneous biopsy, percutaneous transhepatic biliary drainage (PTBD, embolization or endoscopic retrograde cholangiopancreatography. In 13 patients (17 %) the etiology of VAPA was acute or chronic pancreatitis. Further etiologies of VAPA are listed in Table 2.
Table 2.
Etiology of visceral artery pseudoaneurysms.
| Previous medical procedures | 48 (62 %) |
|---|---|
| 1. Pancreatic surgery (including Whipple procedure) | 19 (25 %) |
| 2. Hepatobiliary surgery (transplantation, hepatectomy, cholecystectomy) | 12 (16 %) |
| 3. Interventions (PTBD, embolization, ERCP, biopsy) | 8 (10 %) |
| 4. GI-tract operations (gastrectomy, small bowel resection) | 4 (5 %) |
| 5. Others (Y-Graft of the aorta, renal transplantation) | 8 (10 %) |
| Trauma (spleen, pancreas) | 5 (6,5 %) |
| Septic shock and endocarditis | 6 (8 %) |
| Pancreatitis | 13 (17 %) |
| Others (peptic ulcer disease, segmental arterial mediolysis) | 5 (6,5 %) |
PTBD: Percutaneous transhepatic biliary drainage. ERCP: endoscopic retrograde cholangiopancreatography. GI-tract: gastrointestinal tract.
3.2. Location of VAPA
In the majority of cases VAPA involved the hepatic artery (n = 31, 38 %), followed by the splenic (n = 18, 22 %) and superior mesenteric artery (SMA) (n = 8, 10 %). The coeliac trunk and gastroduodenal artery were each involved six times (7 %).
VAPAs in the hepatic artery were missed in 20/31 cases (65 %) and VAPAs in the splenic artery were missed in 10/18 cases (56 %). All VAPAs in the ileocolic and jejunal artery (each, n = 2) were missed, but none was missed in pancreatic arteries (n = 3) and in the left gastric artery (n = 1). Of the six VAPAs in the coeliac trunk and gastroduodenal artery four were missed (67 %). Six out of 8 VAPAs (75 %) were missed in the SMA, and three of four VAPAs (75 %) were missed in the pancreaticoduodenal artery.
We subdivided the VAPA locations into two groups: those located in major visceral vessels including the splenic artery, the hepatic artery, the coeliac trunk, the left gastric artery and the SMA; and those located in minor, smaller vessels, including the ileocolic and jejunal arteries, the gastroduodenal artery, the small segmental branches of the hepatic artery and branches of the pancreatic arteries. Using this subdivision, we found no significant differences in the rate of VAPA that were missed in major as compared to those missed in minor, smaller vessels (Chi-Square test, p = 0.601).
3.3. Size of VAPA
The overall size of VAPA (n = 81) ranged from 2 to 60 mm (median diameter 9 mm, Table 3). The median size increase of VAPA over one month was 2 mm (range 0–33 mm). The median size of missed VAPA (8 mm) was significantly smaller than that of correctly diagnosed VAPA (median diameter 13 mm, Mann-Whitney U test, p < 0.01).
Table 3.
Localization and size of VAPA.
| Arterial Vessel | |
|---|---|
| Hepatic artery (common, right, left) | 31 (38 %) |
| Splenic artery | 18 (22 %) |
| Superior mesenteric artery | 8 (10 %) |
| Coeliac trunk | 6 (7 %) |
| Ileocolic artery | 2 (2 %) |
| Gastroduodenal artery | 6 (7 %) |
| Pancreaticoduodenal artery | 4 (5 %) |
| Jejunal artery | 2 (2 %) |
| Left gastric artery | 1 (1 %) |
| Pancreatic artery (dorsal, greater, inferior) | 3 (4 %) |
| Maximal diameter of VAPA in CT | Median 9.0 mm Interquartile range 2.0–16 mm |
3.4. Clinical presentation of patients with VAPA
Sixty-one of the 77 patients (79 %) had symptoms upon hospital admission and 16/77 patients (21 %) were asymptomatic, meaning that the latter patients received a CT scan not directly related to the VAPA or received a screening CT in case of sepsis. Out of the 61 patients with symptoms, 57 (74 %) presented with a ruptured VAPA, in four cases (4/77, 5 %) no rupture was detected and abdominal pain was the sole symptom.
Of the 57 patients with ruptured VAPA 8 patients (10 %) had hemodynamic shock, 7 (9 %) had non-specific symptoms such as abdominal pain, and 48 (62 %) showed direct or indirect signs of bleeding with the majority having a reduced hemoglobin count (n = 14, 18 %), hemobilia or bleeding from a PTBD (n = 10, 13 %), or upper/lower gastrointestinal bleeding (n = 11, 14 %).
Of the 45 patients with initially missed diagnosis, 38 (84 %) had a reduced hemoglobin count and 7 (16 %) were asymptomatic, meaning that the VAPA was an incidental finding in follow-up CT performed for reasons not directly related to the pseudoaneurysm. Of the 32 patients (42 %) with a correct initial diagnosis, 22 (69 %) had symptoms while 10 (31 %) were asymptomatic (Table 4). There was no difference in the rate of missed VAPA in symptomatic as compared to that in asymptomatic patients (Chi-Square test, p = 0.255).
Table 4.
Patients’ symptoms and incidence of rupture.
| Symptomatic | 61 (79 %) |
|---|---|
| Rupture | 57 (74 %) |
| Pain | 11 (14 %) |
| Direct and indirect bleeding signs | 48 (62 %) |
| Reduced hemoglobin count | 14 (18 %) |
| Haemobilia and bleeding from PTBD | 10 (13 %) |
| Upper and lower GI-tract bleeding | 11 (14 %) |
| Shock | 8 (10 %) |
| Asymptomatic | 16 (21 %) |
PTBD: Percutaneous transhepatic biliary drainage. GI-tract: gastrointestinal tract.
3.5. Reasons for misdiagnosis of VAPA in CT
Missing contrast phase: In 16 of the 45 cases (36 %) with missed VAPA in CT, no arterial contrast media phase was acquired, making it difficult to differentiate the aneurysm from surrounding tissue, including adjacent veins and/or lymph nodes (Fig. 2). Retrospective analysis of the written indication for CT by referring physicians in this group showed the following: in 6 of these 16 patients (38 %) the clinical indication for CT was abdominal infection/abscess, in 4/16 patients (25 %) the indication was related to tumor progression, in 3/16 patients (19 %) the indication was ileus or leakage of a gastrointestinal tract anastomosis, and in one case (6 %) the indication was abdominal venous thrombosis. In one case (6 %) the clinical question was bleeding, but the CT scan start was delayed because of technical problems, and in one case (6 %) the indication for CT included various unspecific questions (i.e., infection/abscess, ileus, free fluid, bleeding), so the protocol was not focused on bleeding and/or aneurysm search.
Fig. 2.
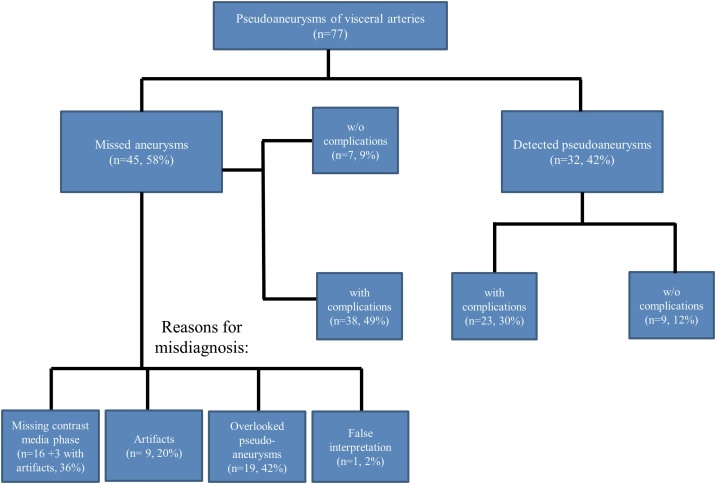
(a, b) 59-year-old male patient with pseudoaneurysm of the common hepatic artery (arrow), which was not diagnosed in initial CT because of missing arterial phase. (a) CT in the portal venous phase through the upper abdomen. (b) Digital subtraction angiography.
(c, d, e) 67-year-old male patient with pseudoaneurysm of the right hepatic artery (arrow), which was not diagnosed in initial CT because of a missing arterial phase and presence of artifacts. (c) CT in the portal venous phase through the upper abdomen in a soft-tissue window. (d) Same CT section with a bone window. (e) Digital subtraction angiography.
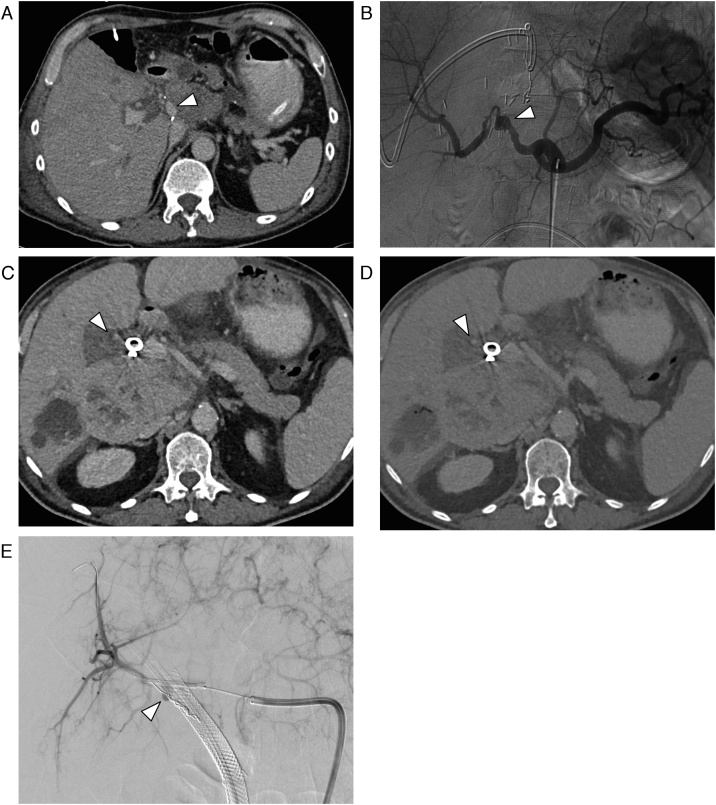
Artifacts: In 9 of the 45 cases (20 %), the localization of the VAPA was directly adjacent to surgical clips, drainages or other interventional foreign bodies (such as a PTBD drain or a coil), hereby making the correct identification of the pseudoaneurysm difficult because of artifacts (Fig. 3). In 3 of these 9 cases an arterial contrast media phase was also not acquired, which further complicated the diagnosis.
Fig. 3.
78-year-old male patient with a pseudoaneurysm of the right hepatic artery (arrow), which was not diagnosed in initial CT because of artefacts from percutaneous transhepatic biliary drainage. (a) CT in the arterial phase through the upper abdomen in a soft-tissue window. (b) Same CT section with a bone window. (c) Digital subtraction angiography.
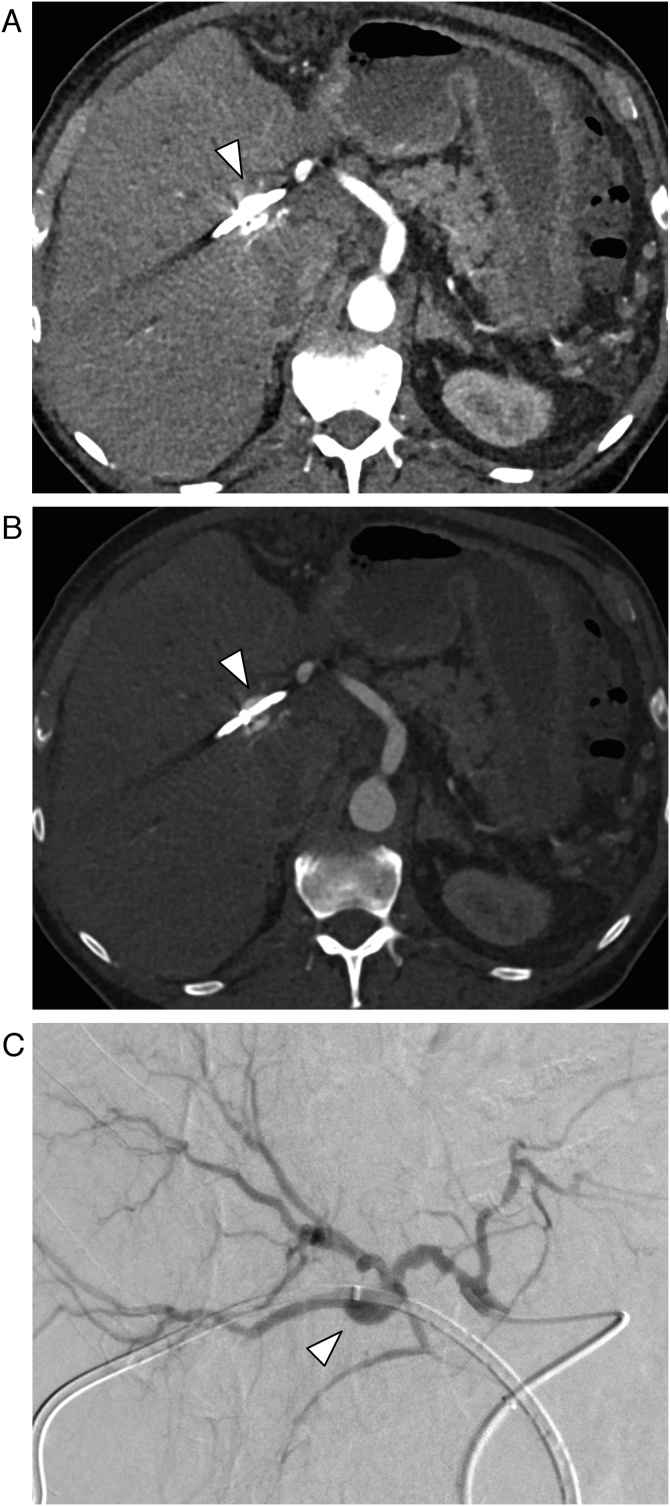
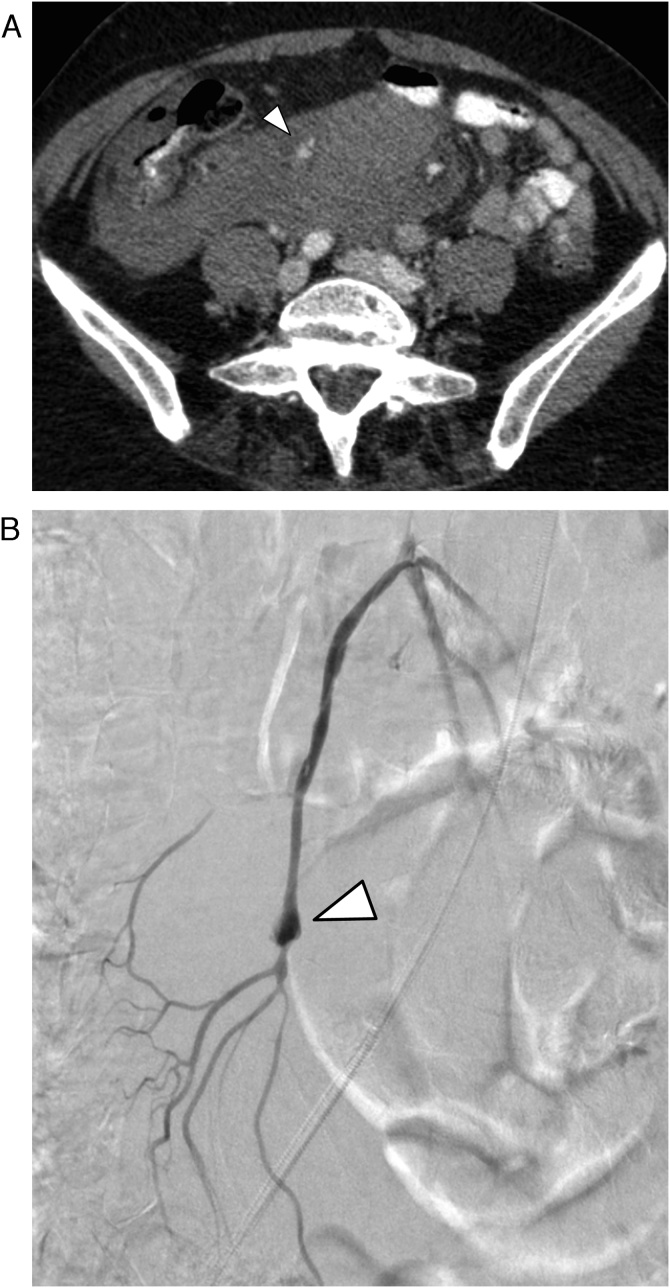
Overlooked diagnosis: In 19 of the 45 patients (42 %) the VAPA was missed (Fig. 4) despite of the presence of multi-phase CT including an arterial phase and in the absence of artifacts.
Fig. 4.
45-year-old male patient with pseudoaneurysm of the superior pancreaticoduodenal artery (arrow), which was overlooked in initial CT. (a) CT in the arterial phase. (b) Digital subtraction angiography.
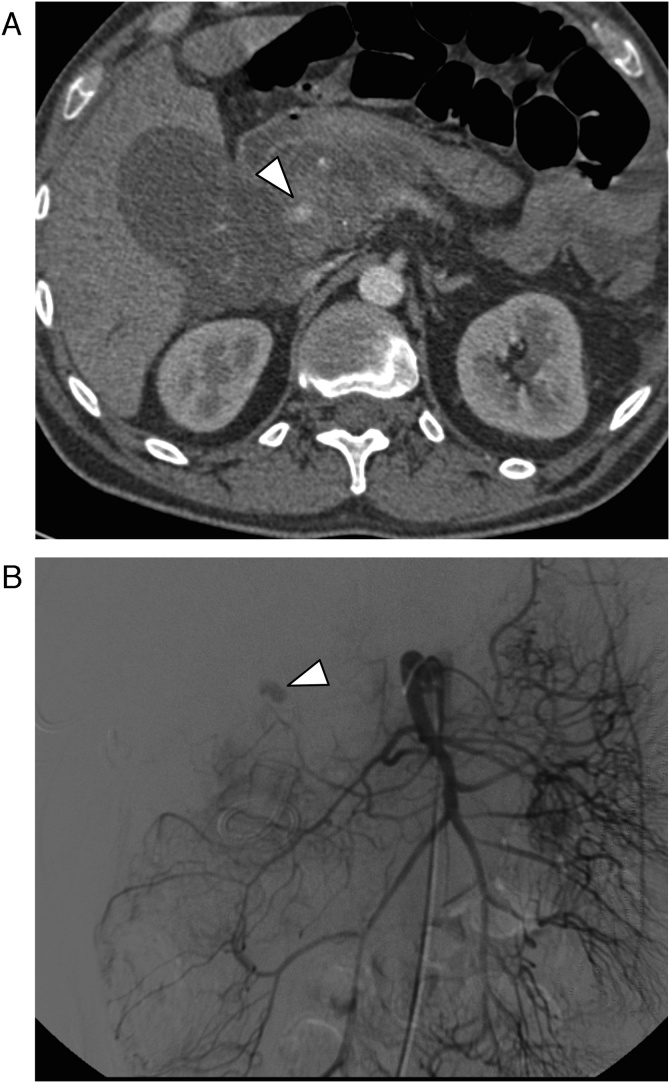
Misinterpretation: In one patient, the attending radiologist misinterpreted the VAPA including contained rupture as a partially enhancing abdominal mass of unknown etiology (Fig. 5).
Fig. 5.
71-year-old female patient with pseudoaneurysm of the ileocolic artery (arrow), which was not diagnosed in initial CT because of false interpretation (the missed ruptured VAPA was described as a partially enhancing mass). (a) CT in the arterial phase through the mesentery. (b) Digital subtraction angiography.
3.6. Follow-up of VAPA
In 62 of the 77 cases (81 %) with VAPA, interventional aneurysm treatment was performed (coil embolization, stent grafting or glue embolization), and one patient (1 %) underwent open surgery. In 14 cases (18 %), patients were treated conservatively or intervention was unsuccessful. Sixty-one patients (79 %) had a satisfactory final outcome without further complications, 4 (5 %) underwent a secondary intervention/surgery, three patients (4 %) had minor complications such as partial spleen/liver infarction, and 9 patients (12 %) died soon after diagnosis (Table 5). Out of the 9 patients with poor outcome, five patients died because of bleeding complications shortly after the intervention or because of an unsuccessful intervention, one patient died three months later because of a new ruptured VAPA, two died of gut ischemia/perforation and one died because of thrombosis.
Table 5.
Management of VAPA and outcome.
| Therapy | |
|---|---|
| Intervention (stentgraft, coiling, glue-embolization) | 62 (81 %) |
| Open surgery | 1 (1 %) |
| Conservative / non successful intervention | 14 (18 %) |
| Outcome | |
| Good | 61 (79 %) |
| Reintervention / Reoperation | 4 (5 %) |
| Exitus | 9 (12 %) |
| Others (spleen/liver infarction, splenectomy) | 3 (4 %) |
4. Discussion
The detection rate of VAPA is increasing owing to the more widespread use of cross-sectional imaging [3]. Also, surgery has become more extensive and various new interventional procedures evolved over the past decade, which also results in an increased prevalence of VAPA. In our study, 48 % of the patients with VAPA had foregoing medical procedures, mostly pancreas (25 %) or hepatobiliary surgery (16 %), and 10 % had previous interventions. The etiology of VAPA in 17 % was acute or chronic pancreatitis. The clinical complexity of the disease is also highlighted by the fact that 73 % of our patients had relevant comorbidities, including hypertension, smoking, acute or chronic nephropathy, and diabetes.
VAPA are potentially life-threatening and missed or delayed diagnosis may worsen the patients’ prognosis. Independent of their associated symptoms or diameter, pseudoaneurysm should always be treated [16]. In our center we treated most patients (81 %) with VAPA through endovascular procedures, while only 1 % underwent open surgery. Most of the patients (79 %) had a good final outcome, 9 % had minor complications or needed further intervention, but 12 % had major complications eventually leading to death.
There have been some studies analyzing the impact of initially misdiagnosed true aneurysms [17,18], but none so far – to our knowledge – analyzed the frequency and reasons for missed or delayed diagnosis of VAPA. We found that more than half (58 %) of our patients had been initially misdiagnosed. The majority of VAPAs occurred in the hepatic (38 %) and splenic artery (22 %). Only 10 % occurred in the SMA and 7 % each in the coeliac trunk and in the gastroduodenal artery. Interestingly, we found no difference between VAPA missed in smaller vessels than those missed in larger vessels, and there was also no difference in the rate of missed VAPA in symptomatic versus asymptomatic patients. As expected, we found that correctly diagnosed VAPA were on average larger than missed ones.
Misdiagnoses were caused by several reasons: in 36 % of the cases the presenting symptoms were not specific and the first assessment with CT was made with a portal-venous phase only (without adding an arterial phase). In these patients the question of referring physicians included always other abdominal diseases which not necessarily requiring an arterial phase of enhancement. There is a known time delay between bleeding and lowering of the hemoglobin count and sometimes laboratory test results are not ready before CT. This highlights the fact that the clinical suspicion of complicated VAPA remains difficult also from a clinical perspective. Thus, radiologists should - based on the previous history of the patient and in particular when it includes foregoing pancreatic and/or hepatic surgery/interventions and diseases - add an arterial phase to the CT protocol independently of the specific clinical question at hand, since an arterial phase CT is the most useful method for detecting pseudoaneurysms and bleeding [19,20].
Twenty percent of our patients with a misdiagnosed VAPA had a surgical drain or coil material within the abdomen, being the cause for artifacts masking the pseudoaneurysm. It is known that metallic implants induce artifacts in CT imaging, thus potentially masking pathologies [21,22]. Thus, implementation of metal artifact reduction software in routine clinical CT protocols appears mandatory for improving the diagnostic capabilities of radiological images [23]. In our study, we found that even changing the window settings (and without applying sophisticated artifact reduction techniques) may help in improving the conspicuity of VAPA detection (see examples in Fig. 2, Fig. 3).
In our patients, 42 % of missed VAPA were overlooked by the attending radiologists. VAPA were often the result of foregoing abdominal surgery and interventions such as Whipple’s operation or liver transplantation [24]. Such patients often have alterations in their normal vessel anatomy with some vessels were clipped, thus making the diagnosis and interpretation of images for less experienced radiologists difficult [25,26]. Knowledge of the normal postoperative anatomy should be therefore enhanced and radiologists should gather further information regarding the type of surgery and the patient’s clinical conditions before interpreting CT [27]. Similar explanations might hold true for the one case with misinterpretation of the VAPA as an enhancing abdominal mass of unknown etiology. Focused and continuous teaching of young radiologists should be done and second readings by more experienced staff should be standard for reducing the number of diagnostic errors [28,29]. In our experience, thick maximum intensity projection images in both axial and coronal planes are particularly helpful for making the diagnosis of VAPA.
The phenomenon of “satisfaction of search” is a well-known type of diagnostic error in radiology, meaning that if an abnormal finding is first detected radiologists often stop searching and other findings might be missed [30]. This is consistent with our study where in all three patients simultaneously presenting with more than one VAPA, at least one pseudoaneurysm was missed. This shows the importance of an accurate review of CT scans even if a major finding has been already detected.
The following study limitations must be acknowledged. First, this retrospective study included only VAPA that were diagnosed as some time-point hereby potentially underestimating their real frequency. There might be a larger number of patients with missed VAPA, who joined another hospital for further treatment, or that were asymptomatic in the further course of the disease. On the other hand, since our hospital is a primary care center for extended abdominal surgery the true frequency of diagnosed and misdiagnosed VAPA could have been overestimated as well. Second, we did not consider patients undergoing MR imaging, since CT is the modality of choice in the emergency setting of our hospital and CTA is known to be the standard non-invasive imaging method for the assessment of the aorta [31] and its branches. Finally, we have no histopathological proof of the diagnosis of pseudoaneurysm in most cases, similar to previous studies [14].
In conclusion, we found a considerable frequency of delayed diagnosis of VAPA. Since clinical and laboratory findings may be unspecific at the time of CT, early and correct radiological diagnosis is crucial to not worsen the prognosis of the patients. Including an arterial contrast media phase to the CT protocol, techniques for artifact reduction and increased awareness of the attending radiologist to the diagnosis of VAPA appear to be the most effective ways to reduce the relatively high misdiagnosis rate.
Authorship conformation form
Please check the following as appropriate:
X All authors have participated in (a) conception and design, or analysis and interpretation of the data; (b) drafting the article or revising it critically for important intellectual content; and (c) approval of the final version.
X This manuscript has not been submitted to, nor is under review at, another journal or other publishing venue.
X The authors have no affiliation with any organization with a direct or indirect financial interest in the subject matter discussed in the manuscript
The following authors have affiliations with organizations with direct or indirect financial interest in the subject matter discussed in the manuscript:
Declaration of Competing Interest
The authors declare that they have no conflicts of interest with the subject of this paper.
References
- 1.Lu M., Weiss C., Fishman E.K., Johnson P.T., Verde F. Review of visceral aneurysms and pseudoaneurysms. J. Comput. Assist. Tomogr. 2015;39(1):1–6. doi: 10.1097/RCT.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 2.Saad N.E., Saad W.E., Davies M.G., Waldman D.L., Fultz P.J., Rubens D.J. Pseudoaneurysms and the role of minimally invasive techniques in their management. Radiographics. 2005;25(Suppl 1):S173–89. doi: 10.1148/rg.25si055503. [DOI] [PubMed] [Google Scholar]
- 3.McDermott V.G., Shlansky-Goldberg R., Cope C. Endovascular management of splenic artery aneurysms and pseudoaneurysms. Cardiovasc. Intervent. Radiol. 1994;17(4):179–184. doi: 10.1007/BF00571531. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal G.A., Johnson P.T., Fishman E.K. Splenic artery aneurysms and pseudoaneurysms: clinical distinctions and CT appearances. AJR Am. J. Roentgenol. 2007;188(4):992–999. doi: 10.2214/AJR.06.0794. [DOI] [PubMed] [Google Scholar]
- 5.Sosogi S., Sato R., Wada R., Saito H., Takauji S., Sakamoto J., Kimura K., Karasaki H., Mizukami Y., Ohta T. Clinical course of conservative management for isolated superior mesenteric arterial dissection. Eur. J. Radiol. Open. 2019;6:192–197. doi: 10.1016/j.ejro.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michael M., Widmer U., Wildermuth S., Barghorn A., Duewell S., Pfammatter T. Segmental arterial mediolysis: CTA findings at presentation and follow-up. AJR Am. J. Roentgenol. 2006;187(6):1463–1469. doi: 10.2214/AJR.05.0281. [DOI] [PubMed] [Google Scholar]
- 7.Alhalabi K., Menias C., Hines R., Mamoun I., Naidu S. Imaging and clinical findings in segmental arterial mediolysis (SAM) Abdom. Radiol. (NY) 2017;42(2):602–611. doi: 10.1007/s00261-016-0887-4. [DOI] [PubMed] [Google Scholar]
- 8.Slavin R.E. Segmental arterial mediolysis: course, sequelae, prognosis, and pathologic-radiologic correlation, Cardiovascular pathology. the official journal of the Society for Cardiovascular Pathology. 2009;18(6):352–360. doi: 10.1016/j.carpath.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Shanley C.J., Shah N.L., Messina L.M. Common splanchnic artery aneurysms: splenic, hepatic, and celiac. Ann. Vasc. Surg. 1996;10(3):315–322. doi: 10.1007/BF02001900. [DOI] [PubMed] [Google Scholar]
- 10.Etezadi V., Gandhi R.T., Benenati J.F., Rochon P., Gordon M., Benenati M.J., Alehashemi S., Katzen B.T., Geisbusch P. Endovascular treatment of visceral and renal artery aneurysms. J. Vasc. Interv. Radiol. 2011;22(9):1246–1253. doi: 10.1016/j.jvir.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Ferrero E., Viazzo A., Ferri M., Robaldo A., Piazza S., Berardi G., Pecchio A., Cumbo P., Nessi F. Management and urgent repair of ruptured visceral artery aneurysms. Ann. Vasc. Surg. 2011;25(7) doi: 10.1016/j.avsg.2011.02.041. 981 e7-11. [DOI] [PubMed] [Google Scholar]
- 12.Nosher J.L., Chung J., Brevetti L.S., Graham A.M., Siegel R.L. Visceral and renal artery aneurysms: a pictorial essay on endovascular therapy. Radiographics. 2006;26(6) doi: 10.1148/rg.266055732. 1687-704; quiz 1687. [DOI] [PubMed] [Google Scholar]
- 13.Rokke O., Sondenaa K., Amundsen S., Bjerke-Larssen T., Jensen D. The diagnosis and management of splanchnic artery aneurysms. Scand. J. Gastroenterol. 1996;31(8):737–743. doi: 10.3109/00365529609010344. [DOI] [PubMed] [Google Scholar]
- 14.Pitton M.B., Dappa E., Jungmann F., Kloeckner R., Schotten S., Wirth G.M., Mittler J., Lang H., Mildenberger P., Kreitner K.F., Oberholzer K., Dueber C. Visceral artery aneurysms: incidence, management, and outcome analysis in a tertiary care center over one decade. Eur. Radiol. 2015;25(7):2004–2014. doi: 10.1007/s00330-015-3599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tessier D.J., Stone W.M., Fowl R.J., Abbas M.A., Andrews J.C., Bower T.C., Gloviczki P. Clinical features and management of splenic artery pseudoaneurysm: case series and cumulative review of literature. J. Vasc. Surg. 2003;38(5):969–974. doi: 10.1016/s0741-5214(03)00710-9. [DOI] [PubMed] [Google Scholar]
- 16.Lagana D., Carrafiello G., Mangini M., Dionigi G., Caronno R., Castelli P., Fugazzola C. Multimodal approach to endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Eur. J. Radiol. 2006;59(1):104–111. doi: 10.1016/j.ejrad.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Azhar B., Patel S.R., Holt P.J., Hinchliffe R.J., Thompson M.M., Karthikesalingam A. Misdiagnosis of ruptured abdominal aortic aneurysm: systematic review and meta-analysis. J. Endovasc. Ther. 2014;21(4):568–575. doi: 10.1583/13-4626MR.1. [DOI] [PubMed] [Google Scholar]
- 18.Smidfelt K., Drott C., Torngren K., Nordanstig J., Herlitz J., Langenskiold M. The impact of initial misdiagnosis of ruptured abdominal aortic aneurysms on lead times, complication rate, and survival. Eur. J. Vasc. Endovasc. Surg. 2017;54(1):21–27. doi: 10.1016/j.ejvs.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Hartnell G.G. Imaging of aortic aneurysms and dissection: CT and MRI. J. Thorac. Imaging. 2001;16(1):35–46. doi: 10.1097/00005382-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Dorffner R., Thurnher S., Youssefzadeh S., Winkelbauer F., Holzenbein T., Polterauer P., Lammer J. Spiral CT angiography in the assessment of abdominal aortic aneurysms after stent grafting: value of maximum intensity projections. J. Comput. Assist. Tomogr. 1997;21(3):472–477. doi: 10.1097/00004728-199705000-00025. [DOI] [PubMed] [Google Scholar]
- 21.Higashigaito K., Angst F., Runge V.M., Alkadhi H., Donati O.F. Metal artifact reduction in pelvic computed tomography with hip prostheses: comparison of virtual monoenergetic extrapolations from dual-energy computed tomography and an iterative metal artifact reduction algorithm in a phantom study. Invest. Radiol. 2015;50(12):828–834. doi: 10.1097/RLI.0000000000000191. [DOI] [PubMed] [Google Scholar]
- 22.Boos J., Fang J., Heidinger B.H., Raptopoulos V., Brook O.R. Dual energy CT angiography: pros and cons of dual-energy metal artifact reduction algorithm in patients after endovascular aortic repair. Abdom. Radiol. (NY) 2017;42(3):749–758. doi: 10.1007/s00261-016-0973-7. [DOI] [PubMed] [Google Scholar]
- 23.Hamie Q.M., Kobe A.R., Mietzsch L., Manhart M., Puippe G.D., Pfammatter T., Guggenberger R. Prototype metal artefact reduction algorithm in flat panel computed tomography - evaluation in patients undergoing transarterial hepatic radioembolisation. Eur. Radiol. 2018;28(1):265–273. doi: 10.1007/s00330-017-4946-1. [DOI] [PubMed] [Google Scholar]
- 24.Volpin E., Pessaux P., Sauvanet A., Sibert A., Kianmanesh R., Durand F., Belghiti J., Sommacale D. Preservation of the arterial vascularisation after hepatic artery pseudoaneurysm following orthotopic liver transplantation: long-term results. Ann. Transplant. 2014;19:346–352. doi: 10.12659/AOT.890473. [DOI] [PubMed] [Google Scholar]
- 25.Lepanto L., Gianfelice D., Dery R., Dagenais M., Lapointe R., Roy A. Postoperative changes, complications, and recurrent disease after Whipple’s operation: CT features. AJR Am. J. Roentgenol. 1994;163(4):841–846. doi: 10.2214/ajr.163.4.7916530. [DOI] [PubMed] [Google Scholar]
- 26.Sandrasegaran K., Maglinte D.D., Howard T.J., Lappas J.C. Surgery for chronic pancreatitis: cross-sectional imaging of postoperative anatomy and complications. AJR Am. J. Roentgenol. 2005;184(4):1118–1127. doi: 10.2214/ajr.184.4.01841118. [DOI] [PubMed] [Google Scholar]
- 27.Anis M., Caroline D. Pitfalls in imaging after gastrointestinal surgery. Semin. Roentgenol. 2015;50(4):328–334. doi: 10.1053/j.ro.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Guly H.R. Diagnostic errors in an accident and emergency department. Emerg. Med. J. 2001;18(4):263–269. doi: 10.1136/emj.18.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikegami Y., Suzuki T., Nemoto C., Tsukada Y., Hasegawa A., Shimada J., Tase C. Establishment and implementation of an effective rule for the interpretation of computed tomography scans by emergency physicians in blunt trauma. World J. Emerg. Surg. 2014;9:40. doi: 10.1186/1749-7922-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim Y.W., Mansfield L.T. Fool me twice: delayed diagnoses in radiology with emphasis on perpetuated errors. AJR Am. J. Roentgenol. 2014;202(3):465–470. doi: 10.2214/AJR.13.11493. [DOI] [PubMed] [Google Scholar]
- 31.Seehofnerova A., Kok M., Mihl C., Douwes D., Sailer A., Nijssen E., de Haan M.J., Wildberger J.E., Das M. Feasibility of low contrast media volume in CT angiography of the aorta. Eur. J. Radiol. Open. 2015;2:58–65. doi: 10.1016/j.ejro.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]