Abstract
The aim of the study was to investigate the effects of tetrandrine combined with acetylcysteine on exercise tolerance, pulmonary function, transforming growth factor-β1 (TGF-β1) and matrix metalloproteinase 7 (MMP-7) in silicosis patients. A retrospective analysis was performed on 149 silicosis patients admitted to the Maternal and Child Health Care Hospital of Zhangqiu District between August, 2015 and September, 2017. Of the 149 patients, 70 patients treated with acetylcysteine comprised the control group, and 79 treated with tetrandrine combined with acetylcysteine constituted the study group. The concentrations of serum TGF-β1 and MMP-7 before and after treatment were detected by enzyme-linked immunosorbent assay (ELISA), and the exercise tolerance and pulmonary function were compared. Chest distress, chest pain, cough, expectoration and dyspnea in the two groups were relieved after treatment, and the improvement rates of chest distress, chest pain and dyspnea in the study group were significantly higher than those in the control group (P<0.05). Before treatment, there was no significant difference in the results of the 6-minute walk test (6MWT) between the two groups (P>0.05). After treatment, the 6MWT in the two groups was significantly increased (P<0.05), and the improvement effect in the study group was more marked than that in the control group (P<0.05). There was no significant difference in the pulmonary function indexes between the two groups before treatment (P>0.05). Before treatment, there was no significant difference in serum TGF-β1 and MMP-7 expression levels between the two groups (P>0.05). By contrast, after treatment, the levels in the two groups were significantly decreased, with the levels in the study group being significantly lower than that the control group (P<0.05). In conclusion, tetrandrine combined with acetylcysteine can improve pulmonary function and exercise tolerance of patients with silicosis by inhibiting the expressions of TGF-β1 and MMP-7, thus improving clinical efficacy.
Keywords: silicosis, tetrandrine, acetylcysteine, exercise tolerance, pulmonary function, TGF-β1, MMP-7
Introduction
Silicosis, one of the most common and severe pneumoconiosis, is characterized by extensive nodular fibrosis in the lungs, which is caused by long-term exposure to silica dust in industry (1). In China, 23,812 new pneumoconiosis cases were reported in 2010, of which 9,870 were silicosis, accounting for 41.45% (2). Silicosis has become a threat to the occupational safety of workers worldwide (3). Therefore, it is particularly important to find an effective treatment.
Tetrandrine is a traditional Chinese medicine extracted from the root tuber of Stephania tetrandra, a type of alkaloid, that can significantly improve the immune function of patients (4,5). Moreover, it is widely used in the treatment of silicosis, with anti-hypertensive, anti-inflammatory and analgesic effects (6). In recent years, acetylcysteine has been used extensively for treating idiopathic pulmonary fibrosis (IPF) and chronic obstructive pulmonary disease (COPD), since it is anti-oxidative stress, anti-fibrosis and anti-inflammatory, and can effectively relieve patients' condition (7,8).
Silicosis is the result of the interaction of many factors and constraints, involving a variety of cells and bioactive substances (9). Transforming growth factor-β1 (TGF-β1), a strong fibrogenic factor, is secreted by a variety of cells, including alveolar macrophages, endothelial cells, fibroblasts, and epithelial cells (10,11). It can regulate cell proliferation and differentiation, stimulate the synthesis and secretion of various cytokines, inflammatory mediators and other active substances, and participate in the formation and degradation of extracellular matrix (11). By establishing silicosis rat models, researchers (12) found that ursolic acid retarded the development of silicosis by reducing the expressions of TGF-β1 and IL-1. Matrix metalloproteinases (MMPs) are potential biomarkers for diagnosing and monitoring the progress of various fibrotic lung diseases (13). MMP-7, the smallest member of the MMPs superfamily, degrades cytoplasmic matrix components (including signal molecules and receptors) and is a key factor in fibrosis (14,15). It has been reported that elevated serum MMP-7 concentration is related to the severity of pulmonary fibrosis and low survival rate of IPF patients (16). The effect of tetrandrine combined with acetylcysteine on serum TGF-β1 and MMP-7 in silicosis patients remains to be further studied. At present, the treatment outcomes of silicosis patients are mainly reflected by pulmonary function indexes (17). The 6-min walk test (6MWT) is a safe detection method that has been widely used to evaluate the degree of pulmonary function decline in patients with pulmonary hypertension, IPF, and COPD (18,19).
The aim of the current study was to mainly evaluate the therapeutic effect of tetrandrine combined with acetylcysteine on silicosis through pulmonary function and exercise tolerance, and explore its effect on the expression levels of serum TGF-β1 and MMP-7.
Materials and methods
General information
A retrospective analysis was performed. The study comprised 149 patients with silicosis who were admitted to the Maternal and Child Health Care Hospital of Zhangqiu District between August, 2015 and September, 2017. Of these patients, 70 patients treated with acetylcysteine comprised the control group, including 51 males and 19 females, with an average age of (52.09±5.78) years. The grade of silicosis was defined according to a previous study (20). There were 42 silicosis patients in stage I, 23 in stage II and 5 in stage III, with a dust exposure duration of (8.01±1.18) years. The remaining 79 patients were treated with tetrandrine combined with acetylcysteine and constituted the study group. This group included 55 males and 24 females, with an average age of (52.87±5.12) years. There were 47 silicosis patients in stage I, 26 in stage II and 6 in stage III, with a dust exposure duration of (7.92±1.21) years.
Inclusion criteria were: patients diagnosed with silicosis by clinical manifestations, high KV chest radiography and pulmonary function examination; patients with complete pathological data and good compliance; patients who actively co-operated; and patients with no blood diseases, diabetes, tumors, hypertension or other diseases. Exclusion criteria were: patients with a history of mental illness; pregnant women; patients complicated with active pulmonary tuberculosis, pneumothorax, respiratory failure and other complications; patients suffering from serious organic diseases such as lung, liver, kidney and brain; and patients with cognitive impairment and unable to communicate normally.
The study was approved by the Ethics Committee of Maternal and Child Health Care Hospital of Zhangqiu District. The subjects were informed of the experimental contents, and their family members signed complete informed consent forms.
Treatment methods
Patients in the control group were given routine treatment and comprehensive treatment, including anti-inflammatory, antiasthmatic and antitussive treatment, and kept away from a dust-exposed work environment. The nutrition and physical exercise were increased to improve their immunity to actively prevent and treat pulmonary tuberculosis and other complications. Acetylcysteine effervescent tablets (600 mg; Zhejiang Conba Biopharmaceutical Co., Ltd.; SFDA approval no. H20057334) were administered twice a day, dissolved in warm boiled water. Twelve days was a course of treatment, one course per month in the first two months, then one course every 2 months, and patients received 4 courses. On the basis of the control group, patients in the study group were given tetrandrine (Zhejiang Zhongyi Pharmaceutical Co., Ltd.; SFDA approval no. H33022163) orally, 60–100 mg each time, three times a day, six days a week. After one course of treatment (3 months), the drug was stopped for 1 month and then repeated for one course.
Serum collection and index detection
In the two groups, 2 ml of fasting peripheral blood collected 1 day before treatment and the morning after treatment were loaded into anticoagulation tubes, respectively, and sent to the clinical laboratory. Then the blood was coagulated for 60 min (20–25°C) and centrifuged at 2,600 × g for 10 min (4°C). The supernatant was collected and stored at −80°C. The concentrations of serum TGF-β1 and MMP-7 in the two groups before and after treatment were detected by enzyme-linked immunosorbent assay (ELISA). The microplate reader was purchased from Bio-Rad Company (450 nm), and the serum TGF-β1 and MMP-7 kits were provided by Shanghai Yuanmu Biotechnology Co., Ltd. (YM-S0090 and YM-S1007). Standard, testing sample and blank wells (without sample and ELISA reagent, the remaining steps were the same) were respectively established. Then, 50 µl of standard sample was added accurately to the microELISA strip plate (Shanghai, Yuanmu Biotechnology, Ltd.) followed by the addition of 40 µl of diluent and 10 µl of sample to the testing sample well. The plate was sealed with a closure plate membrane and incubated at 37°C for 30 min. After uncovering the membrane, the liquid was discarded and patted dry. Each well was filled with PBS washing buffer (Beyotime), which was discarded after standing. This step was repeated five times, then the well was dried. ELISA reagent (50 µl) was added to each well, except for blank well, then incubated and washed. Then, 50 µl of substrate A and 50 µl of substrate B were added to each well, gently mixed and incubated at 37°C for 10 min in the dark. The microplate reader was removed, and 50 µl of termination solution 2 M sulphuric acid (Shanghai, Yuanmu Biotechnology, Ltd.) was added. The optical density (OD) value of each well at 450 mm was measured within 15 min.
Outcome measures
The improvement rates of chest distress, chest pain, cough, expectoration, dyspnea and other clinical symptoms in the two groups were recorded. The 6MWT and lung function indexes [maximal voluntary ventilation (MVV) per minute, percentage of FEV1 and vital capacity percentage (FEV1/FVC%), forced expiratory volume in 1 second (FEV1), peak expiratory flow (PEF) per second] in the two groups were compared. The changes of serum TGF-β1 and MMP-7 before and after treatment were observed.
Statistical treatment
SPSS 19.0 software system (IBM, SPSS, Chicago, IL, USA) was used for statistical analysis of experimental data. Counting data were expressed as [n (%)], and the Chi-square test was used for inter-group comparisons. Measurement data were expressed as (mean ± SD). Inter-group comparisons were conducted by an independent sample t-test, and comparisons between groups were conducted by the paired t-test. P<0.05 indicated statistically significant differences.
Results
Comparison of general data
The general data of patients in the two groups were collected (Table I). There was no significant differences between the two groups in terms of sex, age, average age, dust exposure duration, smoking and silicosis stage (P>0.05).
Table I.
Comparison of general data between the two groups (mean ± SD) [n (%)].
| Characteristic | Study group | Control group | Chi-square test | P-value |
|---|---|---|---|---|
| Sex | 0.189 | 0.663 | ||
| Male | 55 | 51 | ||
| Female | 24 | 19 | ||
| Age (years) | 0.502 | 0.479 | ||
| ≤50 | 44 | 43 | ||
| >50 | 35 | 27 | ||
| Average age (years) | 52.87±5.12 | 52.09±5.78 | 0.874 | 0.384 |
| Dust exposure duration (years) | 7.92±1.21 | 8.01±1.18 | 0.458 | 0.647 |
| Smoking | 0.237 | 0.626 | ||
| Yes | 42 | 40 | ||
| No | 37 | 30 | ||
| Stage | 0.012 | 0.994 | ||
| I | 47 | 42 | ||
| II | 26 | 23 | ||
| III | 6 | 5 |
Comparison of clinical symptoms between the two groups before and after treatment
Cough, expectoration, chest distress, chest pain and dyspnea in the two groups were relieved after treatment (Table II). The improvement rates of chest distress, chest pain and dyspnea in the study group were significantly higher than those in the control group, with statistically significant difference (P<0.05).
Table II.
Comparison of clinical symptoms between two groups before and after treatment/cases.
| Item | Chest distress | Chest pain | Cough | Expectoration | Dyspnea |
|---|---|---|---|---|---|
| Study group (n=79) | |||||
| Before treatment | 69 | 73 | 77 | 72 | 65 |
| After treatment | 7 | 9 | 11 | 13 | 6 |
| Improvement rate | 78.5% | 81.0% | 83.5% | 74.7% | 74.7% |
| Control group (n=70) | |||||
| Before treatment | 63 | 68 | 70 | 67 | 60 |
| After treatment | 19 | 21 | 19 | 15 | 17 |
| Improvement rate | 62.9%a | 67.1%a | 72.9a | 74.3% | 61.4%a |
P<0.05, compared with the study group.
Comparison of exercise tolerance between two groups
Exercise tolerance was compared between the two groups, as shown in Fig. 1. Before treatment, there was no significant difference in the results of 6MWT between the two groups (P>0.05). After treatment, the 6MWT in the two groups was significantly increased (P<0.05), and the improvement effect in the study group was more marked than that in the control group (P<0.05).
Figure 1.
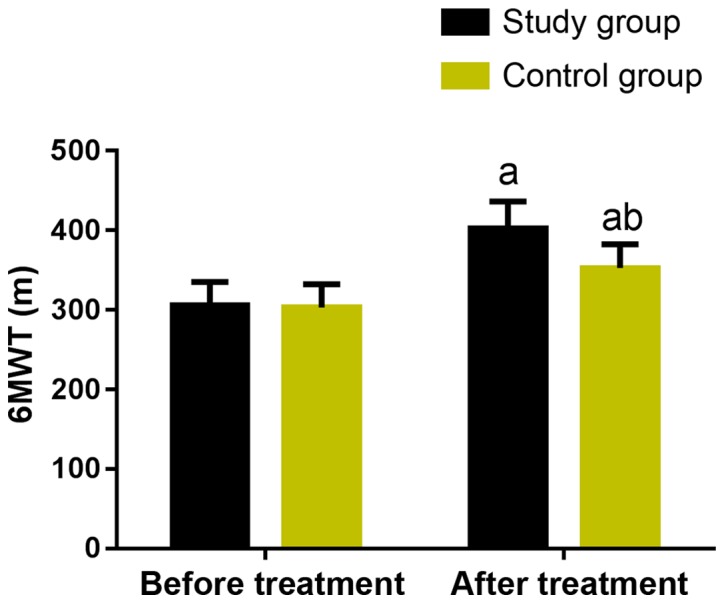
Comparison of exercise tolerance between the two groups. Before treatment, there was no significant difference in the 6MWT between the two groups (P>0.05). After treatment, the 6MWT of the two groups was significantly higher than that before treatment (P<0.05). The change of 6MWT in the study group was more significant than that in the control group (P<0.05). a vs. before treatment, aP<0.05; b vs. the study group after treatment, bP<0.05.
Comparison of pulmonary function between the two groups
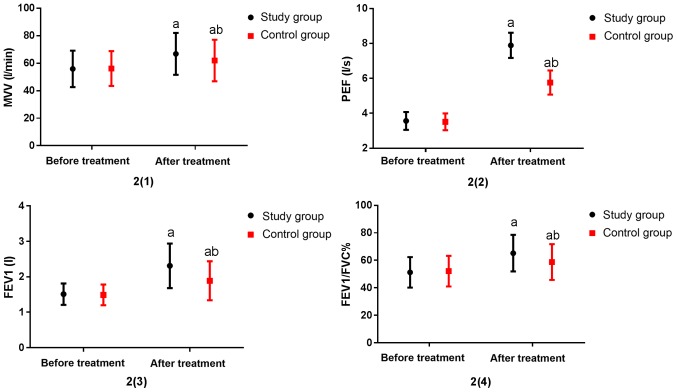
There was no significant difference in pulmonary function indexes between the two groups before treatment (P>0.05). By contrast, the indexes in the two groups were significantly increased after treatment (P<0.05), including MVV, PEF, FEV1, FEV1/FVC%. Specifically, the indexes in the study group were significantly higher than those in the control group (P<0.05) (Fig. 2).
Figure 2.
Comparison of lung function between the two groups. Before treatment, there was no significant difference in lung function between the two groups (P>0.05). After treatment, the lung function indexes MVV, PEF, FEV1, FEV1/FVC% were significantly higher than those before treatment (P <0.05). MVV, PEF, FEV1, FEV1/FVC% in the study group were significantly higher than those in the control group, with statistically significant differences (P<0.05). a vs. before treatment, aP<0.05; b vs. the study group after treatment, bP<0.05.
Changes of serum TGF-β1 and MMP-7 before and after treatment in the two groups
The levels of serum TGF-β1 and MMP-7 in the two groups were measured before and after treatment (Figs. 3 and 4). Before treatment, there was no significant difference in serum TGF-β1 and MMP-7 expression levels between the two groups (P>0.05). However, after treatment, the levels in the two groups were significantly decreased, and the study group was statistically significantly lower than the control group (P<0.05).
Figure 3.
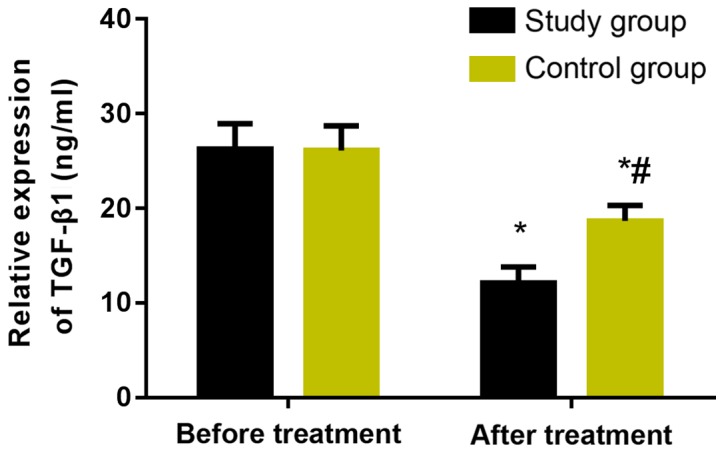
Changes of serum TGF-β1 before and after treatment in the two groups The results of ELISA showed that there was no significant difference in serum TGF-β1 expression level between the two groups before treatment (P>0.05). However, after treatment, the level in the two groups was significantly decreased, and the study group was statistically significantly lower than the control group (P<0.05). *P<0.05, compared with before treatment. #P<0.05, compared with the study group.
Figure 4.
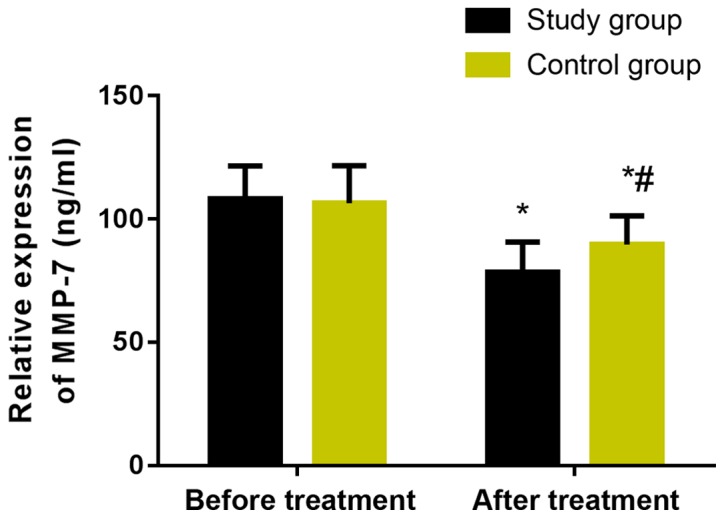
Changes of serum MMP-7 before and after treatment in the two groups. The results of ELISA showed that there was no significant difference in serum MMP-7 expression level between the two groups before treatment (P>0.05). However, after treatment, the level in the two groups was significantly decreased, and the study group was statistically significantly lower than the control group (P<0.05). *P<0.05, compared with before treatment. #P<0.05, compared with the study group.
Discussion
Silicosis is a systemic disease characterized by pulmonary fibrosis, mainly caused by long-term inhalation of dust containing free silica (21). The gradual development of pulmonary fibrosis may lead to gas exchange area reduction, lung function damage, thus resulting in respiratory failure, or even lung cancer in severe cases (22). Therefore, effective clinical treatment should be taken to improve the pulmonary function of patients to prevent the deterioration of the condition.
Tetrandrine is a strong calcium antagonist and affects the transmembrane transport of calcium ions by acting on calcium channels, thus playing a blocking effect on calcium channels of lung fibroblasts. Moreover, it can also promote the loosening and degradation of lung collagen fibers, the disappearance and depolymerization of microtubule structures, thus hindering the transformation of procollagen and preventing pulmonary fibrosis (23). Modern pharmacology shows that tetrandrine improves phagocytosis of macrophages and enhances excretion by acting on collagen macromolecules and combining with them, effectively preventing inflammation and pulmonary fibrosis (24). It has been shown that a free radical-mediated oxidation/antioxidation imbalance plays an important role in the occurrence and development of silica-induced pulmonary fibrosis (25). Acetylcysteine, a precursor of reduced glutathione with an antioxidant effect, can reduce the level of free radicals, antagonize pulmonary fibrosis caused by free radicals, and delay the process of pulmonary fibrosis induced by silicosis (25–27).
The occurrence and progression of silicosis is an interweaving process of inflammation and fibrosis, in which cytokines play an important role (28). TGF-β1 is considered the strongest extracellular matrix precipitation promoter and the most direct cytokine to promote pulmonary fibrosis (29). It may promote the increase of extracellular matrix by up-regulating the expressions of fibronectin and collagen, and may inhibit the degradation of extracellular matrix by increasing the secretion of protease inhibitor and decreasing the secretion of matrix metalloproteinase, eventually participating in the process of pulmonary fibrosis (30). MMP-7 is a matrix metalloproteinase, which has local inflammatory regulatory effects (31,32), and is highly up-regulated in alveolar epithelial cells of idiopathic pulmonary fibrosis (IPF) (32,33). In addition, MMP-7 knockout mice were relatively unaffected by bleomycin (a fibrosis inducer), suggesting that MMP-7 may promote fibrosis in IPF (33). Moreover, MMP-7 may stimulate epithelial cells by lysis of E-cadherin (34) and activate heparin-binding epidermal growth factor-like growth factor (proHB-EGF) by proteolysis, leading to the release of activated HB-EGF and the proliferation of human lung fibroblasts (35), thus promoting pulmonary fibrosis. However, the main mechanism of TGF-β1 and MMP-7 in silicosis patients needs to be further proved.
Findings of this study showed that chest distress, chest pain, cough, expectoration and dyspnea in the two groups were relieved after treatment, and the improvement rates of chest distress, chest pain and dyspnea in the study group were significantly higher than those in the control group, with statistically significant difference (P<0.05). Thus, tetrandrine combined with acetylcysteine effervescent tablets improves the occurrence of adverse respiratory symptoms, and its effect is better than that of single drug. Li et al (36) compared the clinical effects of tetrandrine combined with acetylcysteine effervescent tablets (observation group) and conventional treatment (control group) on silicosis. The results showed that there was no significant difference in respiratory symptoms between the two groups before treatment. After treatment, the symptoms in the two groups were significantly improved, and the improvement rates of cough, expectoration, chest pain, chest distress and other symptoms in the observation group were significantly better than those in the control group. This is similar to the study in this paper, but probably due to the small sample size, there was no significant difference between the two groups in the improvement rates of cough and expectoration. In this study, the exercise tolerance in the two groups was compared. The results showed that there was no significant difference in the results of 6MWT before treatment (P>0.05). After treatment, the 6MWT in the two groups was significantly increased (P<0.05), and the improvement effect in the study group was more marked than that in the control group (P<0.05). Previous findings showed that the 6MWT of patients treated with high-dose N-acetylcysteine was significantly higher than that with conventional dose in treating silicosis (37). Combined with this study, it has been shown that tetrandrine combined with high-dose acetylcysteine can significantly improve exercise tolerance of silicosis patients. There was no significant difference in pulmonary function indexes between the two groups before treatment (P>0.05). After treatment, pulmonary function indexes MVV, PEF, FEV1, FEV1/FVC% in the two groups were significantly increased, and those indexes in the study group were significantly higher than those in the control group. It was also reported that tetrandrine combined with acetylcysteine can significantly improve FVC and FEV and lung ventilation function of silicosis patients (38). Xiao et al (39) used silicosis models to show that tetrandrine combined with acetylcysteine can significantly delay and inhibit the process of pulmonary fibrosis, and the therapeutic effect was better than that of single drug. Therefore, tetrandrine and acetylcysteine can both achieve good therapeutic effects, but the combination is better. It was reported that the high expression of MMP-7 in plasma of IPF patients was positively correlated with the severity of the disease (40), and that the expression of TGF-β1 was upregulated in the peripheral blood of silicosis patients (41). In this study, there was no significant difference in serum TGF-β1 and MMP-7 expression levels between the two groups before treatment. By contrast, after treatment, the levels in the two groups were significantly decreased, and the levels in the study group were significantly lower than those in the control group. This suggests that tetrandrine combined with acetylcysteine can significantly reduce the expressions of TGF-β1 and MMP-7 in serum of silicosis patients. A study found that tetrandrine can inhibit the expression of MMP-2 protein in human umbilical vein endothelial cells (HUVEC) cultured in vitro (42). Combined with the results of this study, it is suggested that tetrandrine may inhibit MMP-7 expression by the same mechanism. The effects of N-acetylcysteine on pulmonary fibrosis, endothelial injury and vasoactive factor in patients with chronic pulmonary heart disease were studied (43), and it was found that N-acetylcysteine can significantly reduce the expression levels of TGF-β1 and MMP-7 in serum of those patients.
The results of the present study mainly explored the curative effect of tetrandrine combined with acetylcysteine on silicosis patients and its influence on TGF-β1 and MMP-7 expressions. The prognosis and influencing factors of silicosis and the mechanism of tetrandrine combined with acetylcysteine in silicosis patients will be further explored, so as to provide more definite references for the pathogenesis, clinical diagnosis and treatment of silicosis. To sum up, tetrandrine combined with acetylcysteine can improve pulmonary function and exercise tolerance of patients with silicosis by inhibiting the expressions of TGF-β1 and MMP-7, thus improving the clinical efficacy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
JZ and YW was responsible for ELISA. SZ analyzed and interpreted the patients' data. JL and HF helped with statistical analysis. JZ wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Maternal and Child Health Care Hospital of Zhangqiu District. Patients who participated in this research, signed the informed consent and had complete clinical data. Signed written informed consents were obtained from the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chen S, Yuan J, Yao S, Jin Y, Chen G, Tian W, Xi J, Xu Z, Weng D, Chen J. Lipopolysaccharides may aggravate apoptosis through accumulation of autophagosomes in alveolar macrophages of human silicosis. Autophagy. 2015;11:2346–2357. doi: 10.1080/15548627.2015.1109765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castranova V, Vallyathan V. Silicosis and coal workers' pneumoconiosis. Environ Health Perspect. 2000;108:675–684. doi: 10.2307/3454404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murtha LA, Morten M, Schuliga MJ, Mabotuwana NS, Hardy SA, Waters DW, Burgess JK, Ngo DT, Sverdlov AL, Knight DA, et al. The role of pathological aging in cardiac and pulmonary fibrosis. Aging Dis. 2019;10:419–428. doi: 10.14336/AD.2018.0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mei L, Chen Y, Wang Z, Wang J, Wan J, Yu C, Liu X, Li W. Synergistic anti-tumour effects of tetrandrine and chloroquine combination therapy in human cancer: A potential antagonistic role for p21. Br J Pharmacol. 2015;172:2232–2245. doi: 10.1111/bph.13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolahian S, Fernandez IE, Eickelberg O, Hartl D. Immune mechanisms in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2016;55:309–322. doi: 10.1165/rcmb.2016-0121TR. [DOI] [PubMed] [Google Scholar]
- 6.Liu T, Liu X, Li W. Tetrandrine, a Chinese plant-derived alkaloid, is a potential candidate for cancer chemotherapy. Oncotarget. 2016;7:40800–40815. doi: 10.18632/oncotarget.8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strieter RM, Mehrad B. New mechanisms of pulmonary fibrosis. Chest. 2009;136:1364–1370. doi: 10.1378/chest.09-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun W, Tang H, Gao L, Sun X, Liu J, Wang W, Wu T, Lin H. Mechanisms of pulmonary fibrosis induced by core fucosylation in pericytes. Int J Biochem Cell Biol. 2017;88:44–54. doi: 10.1016/j.biocel.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Warburton D, Shi W, Xu B. TGF-β-Smad3 signaling in emphysema and pulmonary fibrosis: An epigenetic aberration of normal development? Am J Physiol Lung Cell Mol Physiol. 2013;304:L83–L85. doi: 10.1152/ajplung.00258.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aono Y, Ledford JG, Mukherjee S, Ogawa H, Nishioka Y, Sone S, Beers MF, Noble PW, Wright JR. Surfactant protein-D regulates effector cell function and fibrotic lung remodeling in response to bleomycin injury. Am J Respir Crit Care Med. 2012;185:525–536. doi: 10.1164/rccm.201103-0561OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doyle TJ, Pinto-Plata V, Morse D, Celli BR, Rosas IO. The expanding role of biomarkers in the assessment of smoking-related parenchymal lung diseases. Chest. 2012;142:1027–1034. doi: 10.1378/chest.12-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrison MI, Pither TL, Fisher AJ. Pathophysiology and classification of primary graft dysfunction after lung transplantation. J Thorac Dis. 2017;9:4084–4097. doi: 10.21037/jtd.2017.09.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pei JS, Chou AK, Hsu PC, Tsai CW, Chang WS, Wu MF, Wu MH, Hsia TC, Cheng SP, Bau DT. Contribution of matrix metalloproteinase-7 genotypes to the risk of non-solid tumor, childhood leukemia. Anticancer Res. 2017;37:6679–6684. doi: 10.21873/anticanres.12126. [DOI] [PubMed] [Google Scholar]
- 15.Martinez FJ, Chisholm A, Collard HR, Flaherty KR, Myers J, Raghu G, Walsh SL, White ES, Richeldi L. The diagnosis of idiopathic pulmonary fibrosis: Current and future approaches. Lancet Respir Med. 2017;5:61–71. doi: 10.1016/S2213-2600(16)30325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song JW, Do KH, Jang SJ, Colby TV, Han S, Kim DS. Blood biomarkers MMP-7 and SP-A: predictors of outcome in idiopathic pulmonary fibrosis. Chest. 2013;143:1422–1429. doi: 10.1378/chest.11-2735. [DOI] [PubMed] [Google Scholar]
- 17.Rosas IO, Goldberg HJ, Collard HR, El-Chemaly S, Flaherty K, Hunninghake GM, Lasky JA, Lederer DJ, Machado R, Martinez FJ, et al. A phase II clinical trial of low-dose inhaled carbon monoxide in idiopathic pulmonary fibrosis. Chest. 2018;153:94–104. doi: 10.1016/j.chest.2017.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flaherty KR, Andrei AC, Murray S, Fraley C, Colby TV, Travis WD, Lama V, Kazerooni EA, Gross BH, Toews GB, et al. Idiopathic pulmonary fibrosis: Prognostic value of changes in physiology and six-minute-walk test. Am J Respir Crit Care Med. 2006;174:803–809. doi: 10.1164/rccm.200604-488OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyamoto S, Nagaya N, Satoh T, Kyotani S, Sakamaki F, Fujita M, Nakanishi N, Miyatake K. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2000;161:487–492. doi: 10.1164/ajrccm.161.2.9906015. [DOI] [PubMed] [Google Scholar]
- 20.Ma J, Zhou Y, Li W, Xiao L, Yang M, Tan Q, Xu Y, Chen W. Association between plasma HMGB-1 and silicosis: A case-control study. Int J Mol Sci. 2018;19:4043. doi: 10.3390/ijms19124043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang SX, Liu P, Wei MT, Chen L, Guo Y, Wang RY, Tu ZG, Liang XC. Roles of serum clara cell protein 16 and surfactant protein-D in the early diagnosis and progression of silicosis. J Occup Environ Med. 2007;49:834–839. doi: 10.1097/JOM.0b013e318124a927. [DOI] [PubMed] [Google Scholar]
- 22.Racanelli AC, Kikkers SA, Choi AMK, Cloonan SM. Autophagy and inflammation in chronic respiratory disease. Autophagy. 2018;14:221–232. doi: 10.1080/15548627.2017.1389823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong J, Ma Q. Macrophage polarization and activation at the interface of multi-walled carbon nanotube-induced pulmonary inflammation and fibrosis. Nanotoxicology. 2018;12:153–168. doi: 10.1080/17435390.2018.1425501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raha S, Kim SM, Lee HJ, Lee SJ, Heo JD, Venkatarame Gowda Saralamma V, Ha SE, Kim EH, Mun SP, Kim GS. Essential oil from Korean Chamaecyparis obtusa leaf ameliorates respiratory activity in Sprague Dawley rats and exhibits protection from NF-κB-induced inflammation in WI38 fibroblast cells. Int J Mol Med. 2019;43:393–403. doi: 10.3892/ijmm.2018.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeidler P, Hubbs A, Battelli L, Castranova V. Role of inducible nitric oxide synthase-derived nitric oxide in silica-induced pulmonary inflammation and fibrosis. J Toxicol Environ Health A. 2004;67:1001–1026. doi: 10.1080/15287390490447296. [DOI] [PubMed] [Google Scholar]
- 26.Duru N, Wolfson B, Zhou Q. Mechanisms of the alternative activation of macrophages and non-coding RNAs in the development of radiation-induced lung fibrosis. World J Biol Chem. 2016;7:231–239. doi: 10.4331/wjbc.v7.i4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L, Carron B, Yee HT, Yie TA, Hajjou M, Rom W. Wnt pathway in pulmonary fibrosis in the bleomycin mouse model. J Environ Pathol Toxicol Oncol. 2009;28:99–108. doi: 10.1615/JEnvironPatholToxicolOncol.v28.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Khalil RA. Matrix metalloproteinases, vascular remodeling, and vascular disease. Adv Pharmacol. 2018;81:241–330. doi: 10.1016/bs.apha.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gharib SA, Johnston LK, Huizar I, Birkland TP, Hanson J, Wang Y, Parks WC, Manicone AM. MMP28 promotes macrophage polarization toward M2 cells and augments pulmonary fibrosis. J Leukoc Biol. 2014;95:9–18. doi: 10.1189/jlb.1112587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohammed FF, Smookler DS, Khokha R. Metalloproteinases, inflammation, and rheumatoid arthritis. Ann Rheum Dis. 2003;62:ii43–ii47. doi: 10.1136/ard.62.suppl_2.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/S0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 32.McGuire JK, Li Q, Parks WC. Matrilysin (matrix metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium. Am J Pathol. 2003;162:1831–1843. doi: 10.1016/S0002-9440(10)64318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zuo F, Kaminski N, Eugui E, Allard J, Yakhini Z, Ben-Dor A, Lollini L, Morris D, Kim Y, DeLustro B, et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci USA. 2002;99:6292–6297. doi: 10.1073/pnas.092134099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Datta A, Scotton CJ, Chambers RC. Novel therapeutic approaches for pulmonary fibrosis. Br J Pharmacol. 2011;163:141–172. doi: 10.1111/j.1476-5381.2011.01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Craig VJ, Zhang L, Hagood JS, Owen CA. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2015;53:585–600. doi: 10.1165/rcmb.2015-0020TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SH, Yeo Y, Kim TH, Lee HL, Lee JH, Park YB, Park JS, Kim YH, Song JW, Jhun BW, et al. Korean Interstitial Lung Diseases Study Group Korean guidelines for diagnosis and management of interstitial lung diseases: Part 2. Idiopathic pulmonary fibrosis. Tuberc Respir Dis (Seoul) 2019;82:102–117. doi: 10.4046/trd.2018.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benjamin MM, Khalil RA. Matrix metalloproteinase inhibitors as investigative tools in the pathogenesis and management of vascular disease. Exp Suppl. 2012;103:209–279. doi: 10.1007/978-3-0348-0364-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miao RM, Sun XF, Zhang YY, Wu W, Fang ZH, Zhao R, Zhao DK, Qian GL, Ji J. Clinical efficacy of tetrandrine combined with acetylcysteine effervescent tablets in treatment of silicosis. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2013;31:857–858. (In Chinese) [PubMed] [Google Scholar]
- 39.Xiao Y, Xia H, Zhu L, Li X, Chen R, Yin X, Jiang Z, Feng L, Chen J, Yu M, et al. Study on the therapeutic effects of tetrandrine combined with N-acetylcysteine on experimental silicosis of rats. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2015;33:519–522. (In Chinese) [PubMed] [Google Scholar]
- 40.Ning JX, Zhang LP, Cui Y. Evaluation of clinical efficacy of tretrandrine combined with acetylcysteine effervescent tablets in the treatment of silicosis. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2017;35:298–299. doi: 10.3760/cma.j.issn.1001-9391.2017.04.016. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 41.Miao RM, Zhang XT, Yan YL, He EQ, Guo P, Zhang YY, Zhao DK, Yang ZG, Chen J, Yao MY, et al. Change of serum TGF-beta1 and TNF-alpha in silicosis patients. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2011;29:606–607. (In Chinese) [PubMed] [Google Scholar]
- 42.Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2005;171:1209–1223. doi: 10.1164/rccm.200408-1044SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill MR, Briggs L, Montaño MM, Estrada A, Laurent GJ, Selman M, Pardo A. Promoter variants in tissue inhibitor of metalloproteinase-3 (TIMP-3) protect against susceptibility in pigeon breeders' disease. Thorax. 2004;59:586–590. doi: 10.1136/thx.2003.012690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.