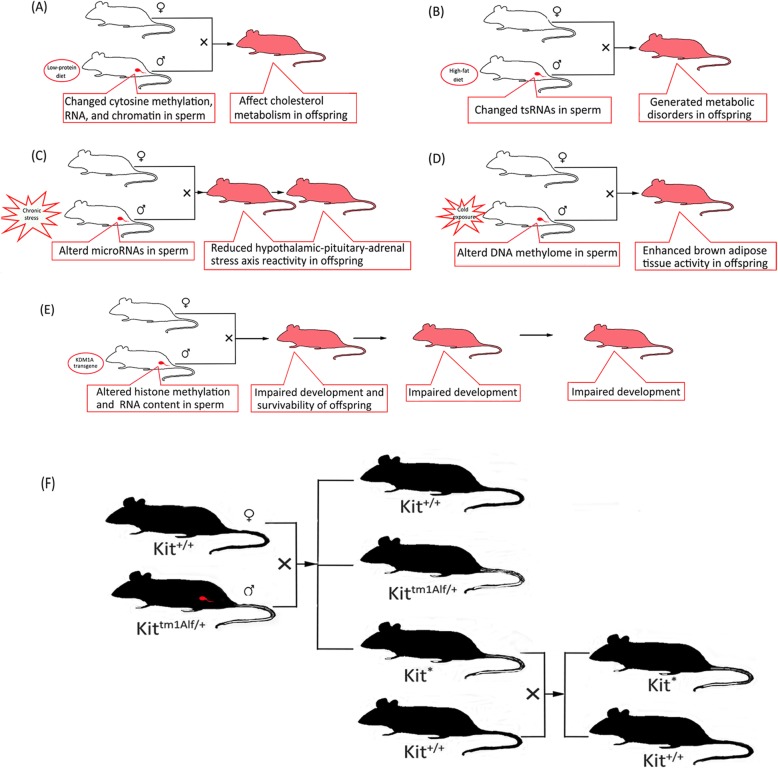
Fig. 1.
Intergenerational and transgenerational epigenetic inheritance via the sperms in mice. a Cytosine methylation, RNA, and chromatin of sperm in male fed a low-protein diet changed, and the offspring exhibited elevated hepatic expression of many genes involved in lipid and cholesterol biosynthesis and decreased levels of cholesterol esters. b Sperm tsRNAs in male fed a high-fat diet exhibited changes in expression profiles and RNA modifications, and this treatment generated metabolic disorders in the F1 offspring. c Chronic paternal stress altered the profiling of sperm microRNAs, which reduced hypothalamic–pituitary–adrenal (HPA) stress axis reactivity in the offspring. d Seasonal or experimental cold exposure induced the epigenetic programming of the sperm such that the offspring harbored hyperactive brown adipose tissue and an improved adaptation to overnutrition and hypothermia. e Disruption of histone methylation in developing sperm by exposure to the KDM1A transgene severely impaired development and survivability of offspring, and the defects occurred in nontransgenic descendants in the absence of KDM1A germline expression. f The genotype of male heterozygous mice Kittm1Alf/+ showed a white tail tip and white feet, and the genotype of mice Kit+/+ showed a full color. The male mice Kittm1Alf/+ were mated with the female mice Kit+/+, and the offspring were of three kinds: offspring (Kit+/+) showed a full color, offspring (Kittm1Alf/+) showed a white tail tip and white feet, and offspring (Kit*) showed a white tail tip and white feet. The genotype of Kit+/+ and Kit* was the same, but the phenotype was different because Kit messenger RNA decreased in the offspring (Kit*). Male mice (Kit*) were mated with female mice (Kit+/+), the offspring still had a white tail tip and white feet, and the degree of this phenomenon reduced