Figure 1.
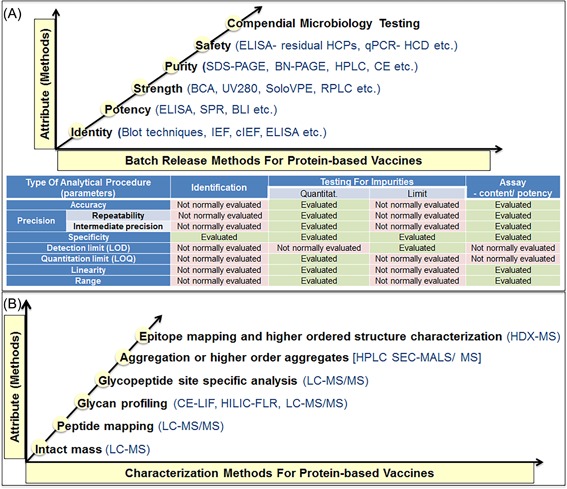
Analytical methods for in‐process and batch release testing of the proteins based vaccines clinical trial material. A, Quality controlled analytical testing for the batch release and stability assessment of the proteins based vaccines and the list of typical parameters which should be considered during the assay validation (or qualification) of analytical method, as per ICH Q2 (R1) guidelines. B, Mass spectrometry based physiochemical characterization testing of the proteins based vaccines
