Figure 5.
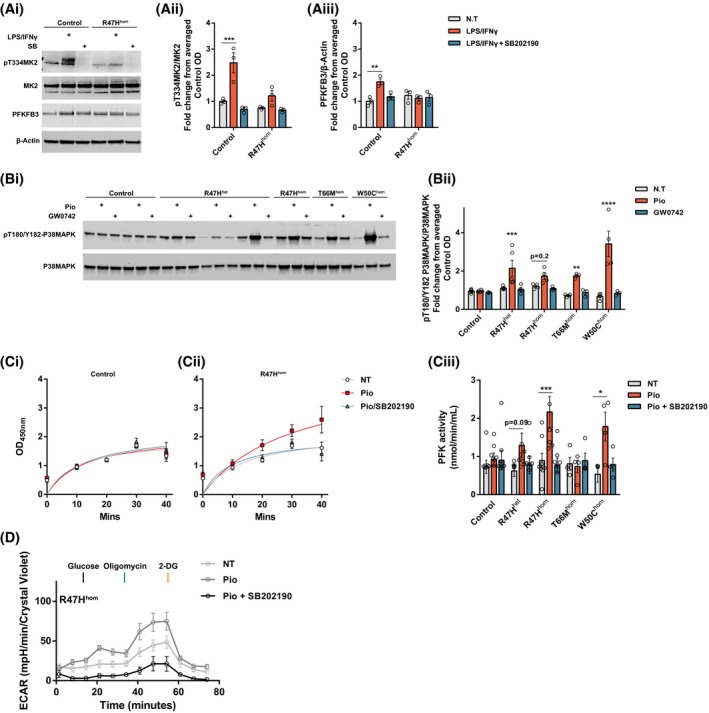
Pioglitazone rescues the energy deficits in TREM2 variant iPS‐Mg through p38‐MAPK and PFKFB3 signaling. Representative western blotting of phospho‐T334‐MK2, total MK2, and PFKFB3 protein levels in control and mutant TREM2 iPS‐Mg after LPS/IFNγ ± SB202190 (Ai) and quantification identified enhanced levels of phospho‐T334‐MK2 (Aii) and PFKFB3 protein (Aiii) after LPS/IFNγ treatment specifically in control lines, when compared to R47Hhom iPS‐Mg, and was dependent on P38MAPK signaling. Representative western blotting of phospho‐T180/Y182‐P38MAPK and total P38MAPK protein levels in control and mutant TREM2 iPS‐Mg after pioglitazone or GW0742 treatment (Bi) and quantification identified the enhanced levels of phospho‐T180/Y182‐P38MAPK after pioglitazone treatment specifically in TREM2 hypomorphic iPS‐Mg lines (Bii). PFK1 enzyme activity is specifically enhanced by pioglitazone in TREM2 hypomorphic lines when compared to control iPS‐Mg, and is dependent on p38MAPK (Ci‐Ciii). Complete traces of the R47Hhom iPS‐Mg glycolytic stress test show that pioglitazone is able to enhance glycolysis and glycolytic function in a P38MAPK‐dependent manner (D). Data are presented as mean ± SEM (n ≥ 3). Statistical significance was addressed using 2‐way ANOVA with Bonferroni’s multiple comparison test to compare LPS/IFNγ, or pioglitazone treatment of each TREM2 variant with the corresponding nontreated group, *P < .05; **P < .01; ***P < .005; ****P < .001
