Figure 3.
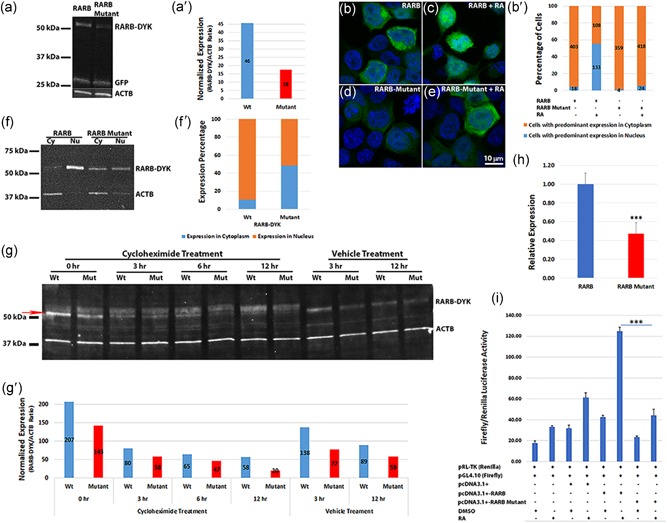
Arg144Gln mutation in RARB results in reduced steady‐state exogenous protein levels, retention in the cytoplasm and affects transcriptional activity. (a) Western blot showing reduced DYK‐tagged RARB‐mutant compared with RARB wild‐type protein in HEK 293‐transfected cells treated with retinoic acid (RA). GFP was used as a control for transfection efficiency and ACTB as a loading control. DYK‐tagged constructs were used for transfection and proteins were detected by DYK, GFP, and ACTB antibodies. (a′) Densitometric analysis of the RARB–DYK bands with normalization to the ACTB. (b–e) Immunofluorescence in HEK 293 cells transfected with wild‐type or mutant RARB–GFP expression constructs followed by RA treatment. Both RARB–GFP and RARB‐mutant‐GFP proteins localized to the cytoplasm in the absence of RA. Upon treatment with RA, wild‐type RARB protein localized to the nucleus, while RARB‐mutant protein was mostly retained in the cytoplasm as revealed by the GFP tag. Scale bar in (e) applies to all images. (b′) Bar graph of the percentage of cells showing predominant localization of the transfected protein in the cytoplasm or the nucleus. Values on the graph indicate the total number of cells counted. Only immunolabeled cells were counted from five different fields. Cells with predominant localization in the cytoplasm (brown); cells with predominant localization in the nucleus (blue). With RA treatment, RARB wild‐type protein showed significantly more nuclear localization compared with the RARB‐mutant protein (p < .01). (f) Nuclear and cytoplasmic fractions separated from transfected HEK 293 cells treated with RA. Wild‐type RARB protein was mostly localized to the nucleus, while RARB‐mutant protein was observed both in the nucleus and in the cytoplasm. (f′) Percentage expression in the nucleus and cytoplasm of RARB–DYK bands from the densitometric analysis. The mutant protein showed reduced expression and localization to the nucleus compared with the wild‐type protein. (g) Protein degradation assay. A T 0 timepoint was harvested before cycloheximide addition (0 hr). The mutant protein was barely visible after 12 hr of cycloheximide treatment, whereas, wild‐type RARB protein was still detected. The red arrow indicates the RARB–DYK band used for densitometric analysis. (g′) Densitometric analysis of the RARB–DYK bands normalized to ACTB. (h) qRT‐PCR of wild‐type and mutant RARB transcripts from the transfected HEK 293 cells normalized to GAPDH and to the neomycin‐resistance gene from the expression vector construct. Expression of the RARB‐mutant transcript was significantly reduced compared with the wild‐type transcript. ***p < .01. (i) Transcriptional activity of RARB wild‐type and mutant proteins. Transcriptional activity is expressed as Firefly/Renilla luciferase activity ratio. RA treatment induced a significant increase in the transcriptional activity of wild‐type RARB but not of RARB‐mutant, ***p < .001. GFP, green fluorescent protein; HEK 293, human embryonic kidney 293; qRT‐PCR, quantitative real‐time polymerase chain reaction
