Figure 4.
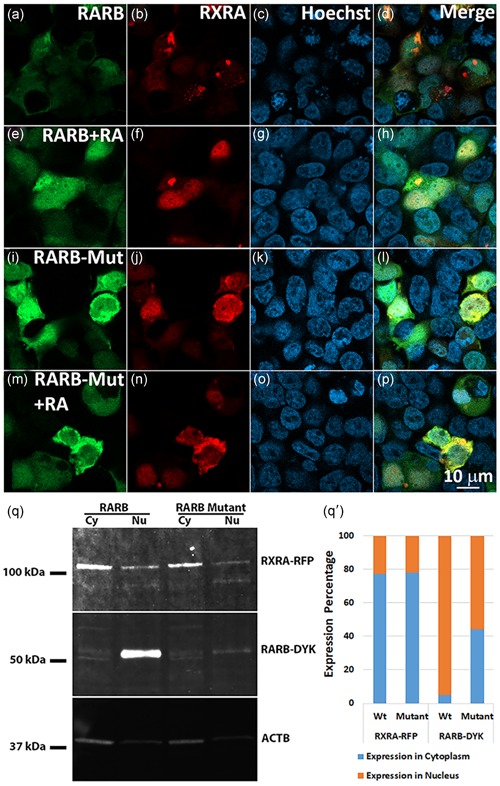
RARB‐mutant protein does not alter the localization of RXR proteins. (a–p) Immunofluorescence of HEK 293 cells treated with DMSO/RA following transfection with GFP‐tagged wild‐type RARB or RARB‐mutant and RFP‐tagged RXRA expression constructs. Wild‐type RARB along with RXRA protein appeared to translocate to the nucleus upon RA treatment whereas partial retention of RXRA protein was seen in the cytoplasm along with the RARB‐mutant protein as revealed by colocalization of GFP and RFP signals from the fusion proteins. Scale bar in (p) applies to all images. (q) Western blots of protein lysates from cytoplasmic and nuclear fractions separated from cells cotransfected with wild‐type or mutant RARB–DYK and RXRA–RFP expression constructs and treated with RA. RXRA localization appeared not affected in cells cotransfected either with RARB wild‐type or mutant expression constructs. However, RARB wild‐type protein was mostly localized to the nucleus while the RARB‐mutant protein was mostly localized to the cytoplasm. (q′) Expression percentage of the RARB–DYK and RXRA–RFP bands from the densitometric analysis. The mutant RARB protein showed reduced expression and translocation to the nucleus compared with the wild‐type protein while the RXRA showed no difference in localization. DMSO, dimethyl sulfoxide; GFP, green fluorescent protein; HEK 293, human embryonic kidney 293; RA, retinoic acid
