Relapse after the completion of induction and consolidation chemotherapy remains a significant cause of mortality in the post‐chemotherapy phase of acute myeloid leukaemia (AML). Several studies have questioned whether AML patients who require two or more courses of induction chemotherapy to attain complete remission (CR) are at increased risk of relapse or death. While studies performed in the 1980’s and 1990’s yielded inconclusive results (Rowe et al., 2010), contemporary studies have identified that needing more than one cycle of induction chemotherapy to achieve CR is a risk factor for relapse and death in younger adult patients (Othus et al., 2019).
Aspects of immunity are relevant to the relapse risk in AML and several immunotherapies aimed at preventing relapse have been developed (Martner et al., 2013; Weinstock et al., 2017; Beyar‐Katz & Gill, 2018; Liu et al., 2019). Immunotherapy with histamine dihydrochloride in conjunction with low‐dose interleukin‐2 (HDC/IL‐2) is approved for relapse prevention in AML patients within the European Union (EU). For this study, we analysed the potential impact of previous induction chemotherapy on the clinical efficacy of HDC/IL‐2.
Three hundred and twenty patients with AML (18–84 years, median 55), who were not eligible for allogeneic stem cell transplantation, were randomly assigned to receive relapse‐preventive immunotherapy with HDC/IL‐2 or no treatment (control group) in a phase III trial. HDC/IL‐2 was initiated in CR after consolidation chemotherapy (Brune et al., 2006). Patients in the treatment arm were scheduled to receive 10 consecutive three‐week cycles of HDC/IL2 with three‐ (cycles 1–3) or six‐week (cycles 4–10) rest periods. In each cycle, these patients received HDC (Noventia Pharma, Milan, Italy) at 0·5 mg and human recombinant low‐dose IL‐2 (aldesleukin; 16 400 IU/kg; Chiron Corporation, Emeryville, CA) subcutaneously twice a day. After 18 months of treatment, patients were monitored for at least 18 additional months. The median follow‐up time was 48 months.
In the CR1 population (n = 261), the number of induction cycles was known in 260 patients, and 203 (78%) of these attained CR after one induction cycle. Among the 57 remaining patients, 47 attained CR after two cycles, while three (n = 7) or four (n = 3) cycles were required to induce CR in 10 patients. Among control patients, there was a non‐significant trend towards inferior clinical outcome in terms of leukemia‐free survival (LFS, defined as the time from inclusion to relapse or death) in those who required more than one induction to attain CR, with a similar trend in younger patients (<60 years old; Figure S1A‐B). In the HDC/IL‐2 arm, outcome was significantly superior in patients who attained CR1 after one induction for all patients as well as for younger patients (Figure S1C‐D). In multivariable analyses, it was found that the number of induction cycles required to attain CR independently predicted outcome for patients in the treatment arm (Table S1).
We compared clinical outcomes for HDC/IL‐2‐treated and control patients who achieved CR after one cycle of induction. In this group, the HDC/IL‐2 arm was significantly superior to the control arm for LFS, with a similar trend for overall survival (OS; Figure 1A‐B). In patients less than 60 years old, treatment with HDC/IL‐2 entailed significantly improved LFS and OS (Figure 1C‐D). The survival advantage for patients in the treatment arm over the control arm remained significant in multivariable analyses (Table 1), correcting for predefined potential confounders (Brune et al., 2006). Treatment with HDC/IL‐2 did not affect LFS or OS in patients who required more than one cycle of induction to attain CR1 (Figure 1E‐F) and was not significantly beneficial in older patients (>60 years old; data not shown). A test of interaction on the effect of HDC/IL‐2 versus controls for LFS supported the finding that it was more pronounced in younger patients who attained CR after one (HR = 0·48) versus more than one (HR = 1·33) cycle of induction (P = 0·04).
Figure 1.
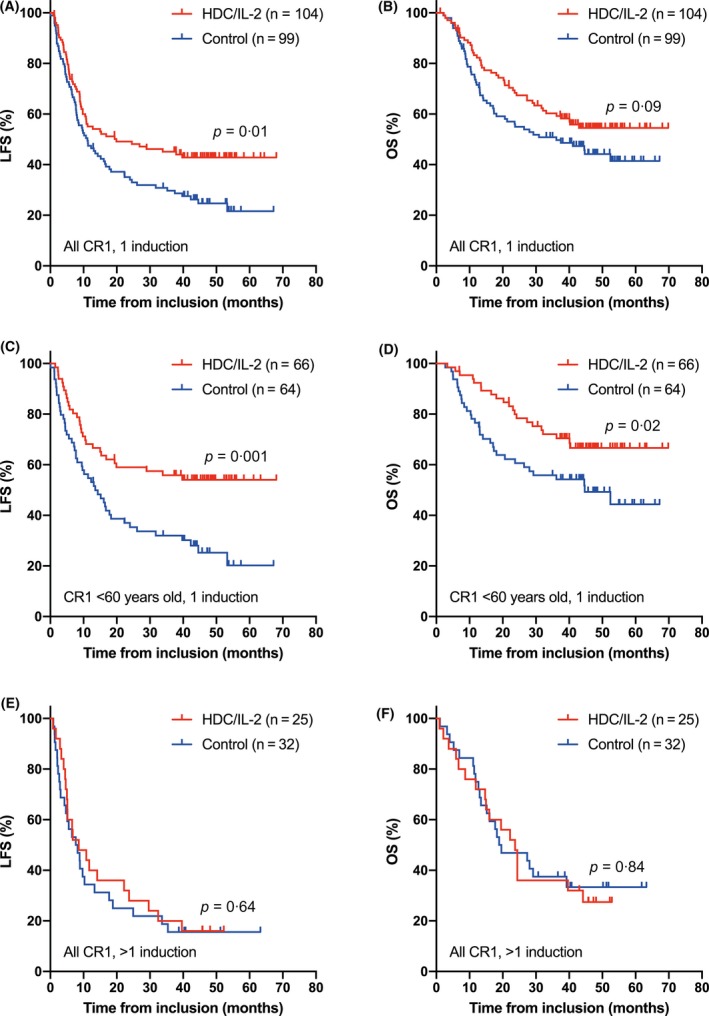
Panels A and B show Kaplan‐Meier plots of LFS and OS in HDC/IL‐2 treated patients or control patients who attained CR1 after the first cycle of induction chemotherapy. Panels C and D show corresponding results in patients <60 years old. Panels E and F show LFS and OS in patients (all ages) who required >1 induction cycle to attain CR1. Statistical analysis was performed by the logrank test.
Table 1.
Cox regression analysis of effects of HDC/IL‐2 treatment versus control on LFS or OS in patients in CR1 who attained CR after one induction cycle. Covariates that tended to affect LFS or OS in univariable analyses (P‐values below 0·1) were included in multivariable analyses.
| Patient groups | Outcome | Univariable analysis | Multivariable analysis* | ||||
|---|---|---|---|---|---|---|---|
| HR† | 95% CI‡ | P‐value | HR | 95% CI | P‐value | ||
| All patients | LFS | 0·65 | 0·46–0·92 | 0·01 | 0·64 | 0·45–0·91 | 0·01 |
| OS | 0·71 | 0·48–1·05 | 0·09 | 0·76 | 0·51–1·14 | 0·18 | |
| <60 years | LFS | 0·48 | 0·30–0·76 | 0·002 | 0·46 | 0·29–0·72 | 0·0008 |
| OS | 0·53 | 0·31–0·92 | 0·02 | 0·49 | 0·28–0·85 | 0·01 | |
| <60 years M4/M5§ | LFS | 0·28 | 0·13–0·62 | 0·002 | 0·28 | 0·13–0·62 | 0·002 |
| OS | 0·28 | 0·10–0·74 | 0·01 | 0·33 | 0·12–0·91 | 0·03 | |
Covariates considered: age; gender (female vs. male); karyotype (favourable vs. intermediate vs. unfavourable); French‐American‐British (FAB) class M0/M1/M5/M6 (yes vs. no); FAB class M2/M3/M4 (yes vs. no); >3 days cytarabine treatment (yes vs. no); secondary AML (yes vs. no); and months from CR1 to random assignment (>6 mo vs. ≤6 mo).
HR = hazard ratio between HDC/IL‐2 and control.
CI = confidence interval.
M4/M5 = French‐American‐British class M4 and M5.
The HDC component of the HDC/IL‐2 regimen aims at countering immunosuppression triggered by reactive oxygen species, formed by the NOX2 enzyme expressed by normal and malignant myeloid cells (Martner et al., 2013; Martner et al., 2019). In AML, functional NOX2 is co‐expressed with histamine H2 receptors (H2R) by leukaemic cells of the M4 and M5 FAB classes (Aurelius et al., 2012a; Aurelius et al., 2012b). We analysed the impact of induction chemotherapy on treatment efficacy in younger patients with FAB M4/M5 AML and observed pronounced efficacy of HDC/IL‐2 versus controls in terms of LFS and OS in patients who attained CR1 after one induction in univariable and multivariable analysis (Figure S2, Table 1).
We conclude that the clinical efficacy of relapse‐preventive immunotherapy with HDC/IL‐2 hinges on the effectiveness of induction chemotherapy. In this regard, our results confirm and extend previous findings in a single‐arm phase IV trial in which 84 AML patients in CR1 received HDC/IL‐2 (Aurelius et al., 2019). In addition, similar results of favourable outcome in patients achieving CR after one versus several cycles of induction were obtained in AML patients undergoing allogeneic transplantation (Walter et al., 2013). Collectively, these results suggest that efficient elimination of leukemic cells during induction treatment favours immune‐mediated clearance of residual leukaemia, thus preventing relapse and death in the post‐chemotherapy phase.
Within the group of patients attaining CR after one induction cycle, our data also point to subgroups of patients, including FAB‐M4/M5 AML, in whom the clinical efficacy of HDC/IL‐2 for relapse prevention is pronounced. These results thus provide means to identify patients who are likely to benefit from immunotherapy and, conversely, patients in whom the therapy is inefficacious. The exploratory nature of these results should be emphasised.
In summary, our results imply that the efficiency of induction chemotherapy independently determines the clinical benefit of relapse‐preventive immunotherapy with HDC/IL‐2 in adult AML patients who are not candidates for allogeneic transplantation. This finding, along with similar results achieved in patients receiving allo‐SCT, suggests that future immunotherapy trials should take the efficiency of induction therapy into account.
Author contributions
Authors MN and AH performed the research. Authors MN, AH, AM, FT and SN analysed the results. Authors MB and KH designed the clinical study. Authors KH, FT and AM wrote the manuscript. All authors approved the manuscript prior to submission.
Conflict of interest
Authors FT, AM and KH hold issued or pending patents protecting the use of NOX2 inhibitors in cancer. Authors MN, AH, MB and SN declare no potential conflicts of interest.
Supporting information
Table S1. Cox regression analysis of the effect of 1 versus >1 induction cycle of chemotherapy on LFS or OS in patients in CR1. Covariates with P‐values below 0.1 in univariable analyses were included in multivariable analyses.
Figure S1. Kaplan‐Meier plots of LFS in all control arm patients in CR1 (A) and in younger control patients (<60 years old, B) who attained CR after 1 versus >1 course of induction chemotherapy. Panels C and D show corresponding results in the HDC/IL‐2 arm. Statistics by the logrank test.
Figure S2. Kaplan‐Meier plots of LFS (A) and OS (B) in younger patients (<60 years old) with AML of FAB M4/M5 classes receiving HDC/IL‐2 or control who attained CR1 after the first course of induction chemotherapy. Statistics by the logrank test.
References
- Aurelius, J. , Martner, A. , Brune, M. , Palmqvist, L. , Hansson, M. , Hellstrand, K. & Thoren, F.B. (2012a) Remission maintenance in acute myeloid leukemia: impact of functional histamine H2 receptors expressed by leukemic cells. Haematologica, 97, 1904–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurelius, J. , Thoren, F.B. , Akhiani, A.A. , Brune, M. , Palmqvist, L. , Hansson, M. , Hellstrand, K. & Martner, A. (2012b) Monocytic AML cells inactivate antileukemic lymphocytes: role of NADPH oxidase/gp91(phox) expression and the PARP‐1/PAR pathway of apoptosis. Blood, 119, 5832–5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurelius, J. , Mollgard, L. , Kiffin, R. , Ewald Sander, F. , Nilsson, S. , Bergh Thoren, F. , Hellstrand, K. & Martner, A. (2019) Anthracycline‐based consolidation may determine outcome of post‐consolidation immunotherapy in AML. Leukemia & Lymphoma, 60, 2771–2778. [DOI] [PubMed] [Google Scholar]
- Beyar‐Katz, O. & Gill, S. (2018) Novel approaches to acute myeloid leukemia immunotherapy. Clinical Cancer Research, 24, 5502–5515. [DOI] [PubMed] [Google Scholar]
- Brune, M. , Castaigne, S. , Catalano, J. , Gehlsen, K. , Ho, A.D. , Hofmann, W.K. , Hogge, D.E. , Nilsson, B. , Or, R. , Romero, A.I. , Rowe, J.M. , Simonsson, B. , Spearing, R. , Stadtmauer, E.A. , Szer, J. , Wallhult, E. & Hellstrand, K. (2006) Improved leukemia‐free survival after postconsolidation immunotherapy with histamine dihydrochloride and interleukin‐2 in acute myeloid leukemia: results of a randomized phase 3 trial. Blood, 108, 88–96. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Bewersdorf, J.P. , Stahl, M. & Zeidan, A.M. (2019) Immunotherapy in acute myeloid leukemia and myelodysplastic syndromes: The dawn of a new era? Blood Reviews, 34, 67–83. [DOI] [PubMed] [Google Scholar]
- Martner, A. , Thoren, F.B. , Aurelius, J. & Hellstrand, K. (2013) Immunotherapeutic strategies for relapse control in acute myeloid leukemia. Blood Reviews, 27, 209–216. [DOI] [PubMed] [Google Scholar]
- Martner, A. , Aydin, E. & Hellstrand, K. (2019) NOX2 in autoimmunity, tumor growth and metastasis. The Journal of Pathology, 247, 151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othus, M. , Estey, E.H. , Garcia‐Manero, G. , Wood, B.L. , Stirewalt, D.L. , Godwin, J.E. , Weick, J.K. , Anderson, J.E. , Appelbaum, F.R. , Erba, H.P. & Walter, R.B. (2019) Second cycle remission achievement with 7+3 and survival in adults with newly diagnosed acute myeloid leukemia: analysis of recent SWOG trials. Leukemia, 33, 554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe, J.M. , Kim, H.T. , Cassileth, P.A. , Lazarus, H.M. , Litzow, M.R. , Wiernik, P.H. & Tallman, M.S. (2010) Adult patients with acute myeloid leukemia who achieve complete remission after 1 or 2 cycles of induction have a similar prognosis: a report on 1980 patients registered to 6 studies conducted by the Eastern Cooperative Oncology Group. Cancer, 116, 5012–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter, R.B. , Buckley, S.A. , Pagel, J.M. , Wood, B.L. , Storer, B.E. , Sandmaier, B.M. , Fang, M. , Gyurkocza, B. , Delaney, C. , Radich, J.P. , Estey, E.H. & Appelbaum, F.R. (2013) Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood, 122, 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock, M. , Rosenblatt, J. & Avigan, D. (2017) Dendritic cell therapies for hematologic malignancies. Molecular Therapy ‐ Methods & Clinical Development, 5, 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cox regression analysis of the effect of 1 versus >1 induction cycle of chemotherapy on LFS or OS in patients in CR1. Covariates with P‐values below 0.1 in univariable analyses were included in multivariable analyses.
Figure S1. Kaplan‐Meier plots of LFS in all control arm patients in CR1 (A) and in younger control patients (<60 years old, B) who attained CR after 1 versus >1 course of induction chemotherapy. Panels C and D show corresponding results in the HDC/IL‐2 arm. Statistics by the logrank test.
Figure S2. Kaplan‐Meier plots of LFS (A) and OS (B) in younger patients (<60 years old) with AML of FAB M4/M5 classes receiving HDC/IL‐2 or control who attained CR1 after the first course of induction chemotherapy. Statistics by the logrank test.


