Table 3.
Substrate scope of azetidine-2-carboxylic acids 5 obtained via palladium-catalyzed hydrogenation of donor–acceptor azetines 3.
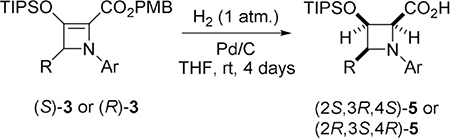 | ||||||
|---|---|---|---|---|---|---|
| Entry[a] | R | Ar | 5 | Yield [%][b] | dr[c] | ee [%][d] |
| 1 | Et | 4-Me-C6H4 | 5 fl | 88 | > 20:1 | 80 |
| 2 | Et | C6H5 | 5 fa | 94 | > 20:1 | 88 |
| 3 | Et | 4-CN-C6H4 | 5 fo | 93 | > 20:1 | 96 |
| 4[e] | Et | 4-CF3-C6H4 | 4 fi | 86 | > 20:1 | 97 |
| 5 | Et | 3-Me-C6H4 | 5 fk | 93 | > 20:1 | 86 |
| 6 | Et | 3-OMe-C6H4 | 5 fj | 90 | > 20:1 | 88 |
| 7 | Et | 4-Et-C6H4 | 5 fm | 87 | > 20:1 | 84 |
| 8 | Et | 2,4-diF-C6H3 | 5 fn | 89 | > 20:1 | 87 |
| 9 | Et | 4-F-C6H4 | 5 fg | 95 | > 20:1 | 99 |
| 10 | Et | 3-F-C6H4 | 5 fc | 92 | > 20:1 | 99 |
| 11 | Et | 2-F-C6H4 | 5 fh | 92 | > 20:1 | 90 |
| 12 | Me | 3-F-C6H4 | 5 ec | 95 | > 20:1 | 90 |
| 13 | octyl | 3-F-C6H4 | 5 gc | 95 | > 20:1 | 99 |
Reactions were carried out on a 0.25 mmol scale of azetine-2-carboxylate 3 in 4.0 mL of THF at room temperature with Pd on activated charcoal (2 wt. % of Pd metal) under H2 (1 atm) for 4 days.
Isolated yields after flash-chromatography are reported.
Determined from the 1H NMR spectra of purified compounds.
Enantiomeric excess is reported based on the ee values of corresponding azetines 3 and confirmed by HPLC analyses.
PMB-ester was isolated.
