Figure 6.
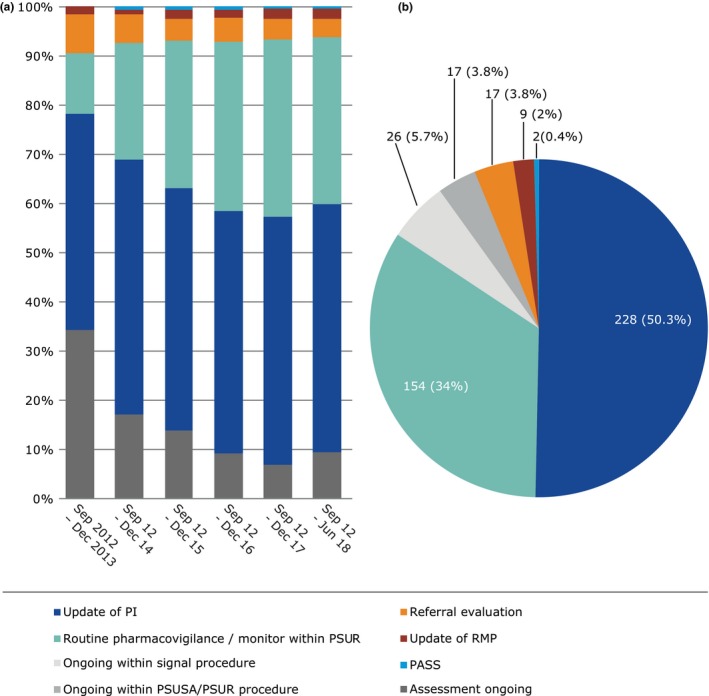
Outcomes of signals prioritized and analyzed at the Pharmacovigilance Risk Assessment Committee in the past 6 years. (a) Per year (b) September 2012 to June 2018. Note for a, 2012 data is merged with 2013 due to the low numbers. PASS, Post Authorization Safety Study; PI, Product Information; PSUR, Periodic Safety Update Report; PSUSA, Periodic Safety Update Single Assessment; Referral, in accordance with the Articles 31 and 107i of Directive 2001/83/EC(4) and Article 20 of Regulation (EC) No. 726/2004; RMP, Risk Management Plan.
