Figure 1.
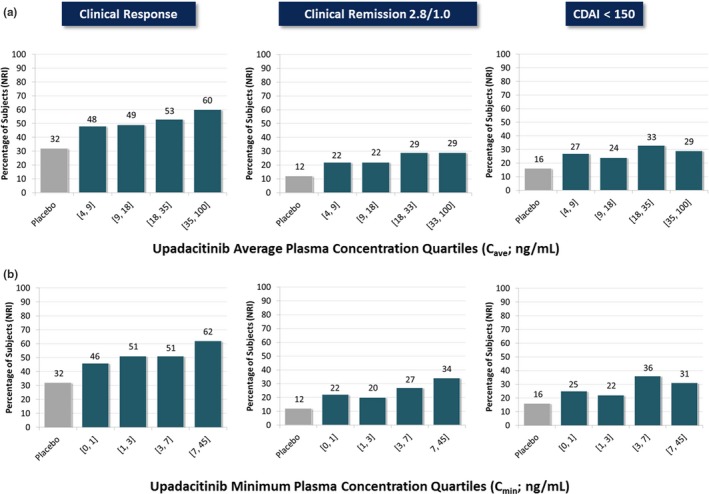
Observed relationships between upadacitinib (a) average plasma concentration during a dosing interval (Cave) or (b) minimum plasma concentration (Cmin) in the phase II CELEST study and the percentages of subjects achieving clinical end points at week 16. CDAI, Clinical Disease Activity Index; NRI, nonresponder imputation. [Colour figure can be viewed at https://www.wileyonlinelibrary.com]
