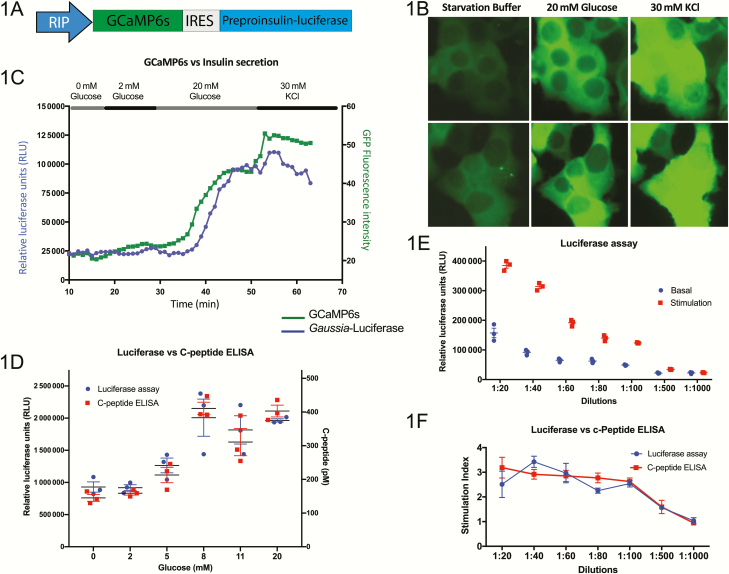
Figure 1.
Generation of a human pancreatic β-cell line with the capability to measure insulin secretion and calcium flux. (A) Schematic representation of the lentiviral construct containing transgenes for GCaMP6s and preproinsulin–Gaussia luciferase, separated by an internal ribosome entry site (IRES) and driven by the rat insulin promoter (RIP). (B) Live-cell imaging of transgenic EndoC-βH1 cells shows an increase in GFP intensity in response to sequential exposure to glucose and KCl (n = 3, representative images from 2 experiments are shown). (C) Measurement of calcium flux by GFP intensity and insulin secretion by luciferase signal of the transgenic EndoC-βH1 line in a perfusion system (n = 3, representative tracking from a single experiment is shown). (D) Luciferase levels show a strong correlation to C-peptide levels measured by ELISA in the transgenic EndoC-βH1 line as a measure of insulin secretion in response to a range of glucose concentrations (n = 3). (E) Serial dilutions measuring luciferase content of the media of transgenic EndoC-βH1 cells under basal (2 mM glucose) and stimulated (20 mM glucose) conditions, showing a sensitivity by limiting dilution (n = 5). (F) Direct comparison of the luciferase assay and human C-Peptide ELISA used to measure the stimulation index (levels at 20 mM glucose over those at 2 mM glucose) of insulin secretion demonstrates the accuracy of measuring luciferase as a surrogate for insulin secretion (n = 5).