Figure 1.
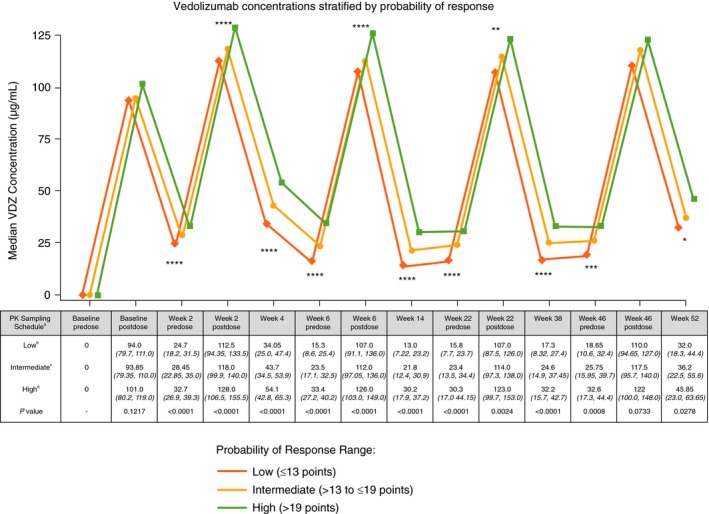
GEMINI 2 clinical trial 52‐week vedolizumab serum drug concentrations stratified by CDST. aAll values in table are median VDZ concentration (µg/mL) (IQR); post‐dose concentration was measured 2 h after dosing. bLow probability; ≤13 points in CDST model at baseline. cIntermediate probability; >13 to ≤19 points in CDST model at baseline. dHigh probability; >19 points in CDST model at baseline. ****P < 0.0001, ***P < 0.001, **P < 0.01, and *P < 0.05. Bolded P values are statistically significant. CDST, clinical decision support tool; IQR, interquartile range; PK, pharmacokinetics; VDZ, vedolizumab
